Abstract
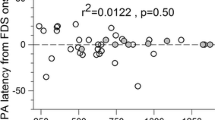
In the present study, we tested a hypothesis that the rhythm generator in humans keeps the rhythm of periodic motor output during brief inactivation of the pattern generator. This investigation was made through testing whether the step rhythm was reset after an interruptive event. A reset of the step rhythm was defined as an observation that the step re-emerges at random timing after an interruptive event regardless of the step rhythm before the interruption. This observation reflects an intermission of rhythm-keeping activity. Healthy participants stepped on a platform that could translate forward or backward. They continued stepping after the platform translation (non-stop session) or stopped briefly after the translation before resuming step with their own timing (stop session). In the non-stop session, the second step after the platform translation appeared at the integer multiple of the pre-existing step period in most participants, indicating that step rhythm was not reset. This finding indicates that postural perturbation does not interfere the rhythm-keeping activity. In the stop session, the step immediately after the intermission of stepping appeared at random time regardless of the step rhythm before the intermission in most participants. The actual side of the first step after the intermission was consistent with the predicted first step side at a 0.5 probability. Those findings indicate that step rhythm is reset after brief intermission of stepping, and contradict with the hypothesis that the activity of the rhythm generator is maintained, while the pattern generator is temporally inactive during a brief intermission of periodic motor output. This analysis could help to determine whether rhythm-keeping activity is inactivated by an interruptive event during periodic motor activity.






Similar content being viewed by others
References
Brown TG (1911) The intrinsic factors in the act of progression in the mammal. Proc Royal Soc London. Series B, containing papers of a biological character, 84(572):308–319. doi:10.1098/rspb.1911.0077
Burke RE, Degtyarenko AM, Simon ES (2001) Patterns of locomotor drive to motoneurons and last-order interneurons: clues to the structure of the CPG. J Neurophysiol 86:447–462
Conway BA, Hultborn H, Kiehn O (1987) Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res 68:643–656. doi:10.1007/BF00249807
Dietz V, Zijlstra W, Duysens J (1994) Human neuronal interlimb coordination during split-belt locomotion. Exp Brain Res 101:513–520. doi:10.1007/BF00227344
Do MC, Breniere Y, Brenguier P (1982) A biomechanical study of balance recovery during the fall forward. J Biomech 15:933–939. doi:10.1016/0021-9290(82)90011-2
Do MC, Schneider C, Chong RKY (1999) Factors influencing the quick onset of stepping following postural perturbation. J Biomech 32:795–802. doi:10.1016/S0021-9290(99)00067-6
Duysens J (1977) Reflex control of locomotion as revealed by stimulation of cutaneous afferents in spontaneously walking premammillary cats. J Neurophysiol 40:737–751
Duysens J (2006) How deletions in a model could help explain deletions in the laboratory. J Neurophysiol 95:562–565
Forssberg H, Grillner S, Halbertsma J, Rossignol S (1980) The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol 108:283–295. doi:10.1111/j.1748-1716.1980.tb06534.x
Frigon A, Rossignol S (2006) Experiments and models of sensorimotor interactions during locomotion. Biol Cybern 95:607. doi:10.1007/s00422-006-0129-x
Frigon A, Barrière G, Fénélon K, Yakovenko S (2007) Conceptualizing the mammalian locomotor central pattern generator with modelling. J Physiol 580:363–364. doi:10.1113/jphysiol.2007.129064
Grillner S (1981) Control of locomotion in bipeds, tetrapods, and fish. In: Brookhart JM, Mountcastle VB (eds) Handbook of Physiology. American Physiological Society, pp 1179–236. doi: 10.1002/cphy.cp010226
Grillner S, Zangger P (1979) On the central generation of locomotion in the low spinal cat. Exp Brain Res 34:241–261. doi:10.1007/BF00235671
Guertin P, Angel MJ, Perreault MC, McCrea DA (1995) Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. J Physiol 487(Pt 1):197–209
Hultborn H, Conway BA, Gossard JP, Brownstone R, Fedirchuk B, Schomburg ED, Enríquez-Denton M, Perreault MC (1998) How do we approach the locomotor network in the mammalian spinal cord? Ann N Y Acad Sci 860:70–82. doi:10.1111/j.1749-6632.1998.tb09039.x
Ivanenko YP, Poppele RE, Lacquaniti F (2009) Distributed neural networks for controlling human locomotion: lessons from normal and SCI subjects. Brain Res Bullet 78:13–21
Kagawa T, Ohta Y, Uno Y (2011) State-dependent corrective reactions for backward balance losses during human walking. Hum Mov Sci 30:1210–1224
Kjaerulff O, Kiehn O (1997) Crossed rhythmic synaptic input to motoneurons during selective activation of the contralateral spinal locomotor network. J Neurosci 17:9433–9447
Kobayashi M, Nomura T, Sato S (2000) Phase-dependent response during human locomotion to impulsive perturbation and its interpretation based on neural mechanism. Jpn J Med Electron Biol Eng 38:20–32 (in Japanese)
Kojima S, Nakajima Y, Takada J (2008) Kinematics of the compensatory step by the trailing leg following an unexpected forward slip while walking. J Physiol Anthropol 27:309–315. doi:10.2114/jpa2.27.309
Lafreniere-Roula M, McCrea DA (2005) Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol 94:1120–1132. doi:10.1152/jn.00216.2005
Lee PY, Gadareh K, Bronstein AM (2014) Forward–backward postural protective stepping responses in young and elderly adults. Hum Mov Sci 34:137–146
Lundberg A (1981) Half-centers revisited. In: J Szentagothai, M Palkovits and J Hamori (eds) Motion and organization principle, vol. 1, Regulatory Function of the CNS. Budapest, Pergamon Akademiai Kiado, pp 155–167
Marigold DS, Patla AE (2002) Strategies for dynamic stability during locomotion on a slippery surface: effects of prior experience and knowledge. J Neurophysiol 88:339–353. doi:10.1152/jn.00691.2001
McCrea DA, Rybak IA (2008) Organization of mammalian locomotor rhythm and pattern generation. Brain Res Rev 57:134–146. doi:10.1016/j.brainresrev.2007.08.006
McIlroy WE, Maki BE (1996) Age-related changes in compensatory stepping in response to unpredictable perturbations. J Gerontol A Biol Sci Med Sci 51:M289–M296. doi:10.1523/ENEURO.0069-15.2015
Nessler JA, Spargo T, Craig-Jones A, Milton JG (2016) Phase resetting behavior in human gait is influenced by treadmill walking speed. Gait Posture 43:187–191
Potocanac Z, Pijnappels M, Verschueren S, van Dieën J, Duysens J (2016) Two-stage muscle activity responses in decisions about leg movement adjustments during trip recovery. J Neurophysiol 115:143–156
Rossignol S, Saltiel P, Perreault M-C, Drew T, Pearson K, Bélanger M (1993) Intralimb and interlimb coordination in the cat during real and fictive rhythmic motor programs. Semin Neurosci 5(1):67–75
Rossignol S, Dubuc R, Gossard JP (2006) Dynamic sensorimotor interactions in locomotion. Physiol Rev 86:89–154
Rybak IA, Lafreniere-Roula M, Shevtsova NA, McCrea DA (2004) A two layer model of the spinal locomotor CPG: insights from deletions of activity during fictive locomotion. In Soc Neurosci Abstr 34:883–884
Rybak IA, Shevtsova NA, Lafreniere-Roula M, McCrea DA (2006) Modelling spinal circuitry involved in locomotor pattern generation: insights from deletions during fictive locomotion. J Physiol 577:617–639. doi:10.1113/jphysiol.2006.118703
Schomburg ED, Petersen N, Barajon I, Hultborn H (1998) Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Exp Brain Res 122:339–350. doi:10.1007/s002210050522
Yamasaki T, Nomura T, Sato S (2003) Phase reset and dynamic stability during human gait. Biosystems 71:221–232
Zehr EP, Duysens J (2004) Regulation of arm and leg movement during human locomotion. Neuroscientist 10:347–361
Zehr EP, Komiyama T, Stein RB (1997) Cutaneous reflexes during human gait: electromyographic and kinematic responses to electrical stimulation. J Neurophysiol 77:3311–3325
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 17K01522.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Hiraoka, K., Kinoshita, A., Kunimura, H. et al. Postural perturbation does not reset stepping rhythm in humans, but brief intermission does. Exp Brain Res 235, 3561–3572 (2017). https://doi.org/10.1007/s00221-017-5084-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-5084-3