Abstract
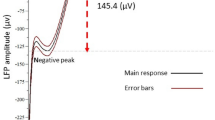
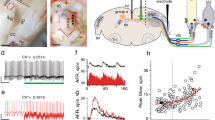
The parafascicular nucleus (PFN) of the thalamus is a primary structure in the feedback circuit of the basal ganglia-thalamo-cortical system, as well as in the neural circuit of the vestibulo-thalamo-striatal pathway. We investigated the characteristics of the functional connectivity between the peripheral vestibular system and the PFN in rats. A single electrical stimulation was applied to the horizontal semicircular canal nerve in the peripheral vestibular end-organs. This resulted in polysynaptic local field potentials (LFPs) in the PFN, which were composed of long-lasting multiple waves. The LFPs were prominently seen contralateral to the stimulation site. The PFN LFPs were suppressed by transient chemical de-afferentation of peripheral vestibular activity using a 5% lidocaine injection into the middle ear. The spontaneous firing rate of the single units increased after electrical stimulation to the horizontal canal nerve in a frequency-dependent manner. The induction of cFos protein was more prominent in the contralateral PFN than in the ipsilateral PFN following horizontal semicircular canal nerve stimulation. The functional vestibulo-parafascicular connection is a neural substrate for the transmission of vestibular sensory information to the basal ganglia.







Similar content being viewed by others
References
Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M (2001) Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI). J Neurophysiol 85:886–899
Blum PS, Gilman S (1979) Vestibular, somatosensory, and auditory input to the thalamus of the cat. Exp Neurol 65:343–354
Bottini G, Sterzi R, Paulesu E et al (1994) Identification of the central vestibular projections in man: a positron emission tomography activation study. Exp Brain Res 99:164–169
Deecke LSD, Fredrickson JM (1974) Nucleus ventroposterior inferior (VPI) as the vestibular thalamic relay in the rhesus monkey. I. Field potential investigation. Exp Brain Res 20(1):88–100
Jouve L, Salin P, Melon C, Kerkerian-Le Goff L (2010) Deep brain stimulation of the center median-parafascicular complex of the thalamus has efficient anti-parkinsonian action associated with widespread cellular responses in the basal ganglia network in a rat model of Parkinson’s disease. J Neurosci 30:9919–9928. doi:10.1523/JNEUROSCI.1404-10.2010
Kilpatrick IC, Phillipson OT (1986) Thalamic control of dopaminergic functions in the caudate-putamen of the rat—I. The influence of electrical stimulation of the parafascicular nucleus on dopamine utilization. Neuroscience 19:965–978
Kim MS, Hyo Kim J, Kry D et al (2003) Effects of acute hypotension on expression of cFos-like protein in the vestibular nuclei of rats. Brain Res 962:111–121
Kincaid AE, Penney JB Jr, Young AB, Newman SW (1991) The globus pallidus receives a projection from the parafascicular nucleus in the rat. Brain Res 553:18–26
Kusnoor SV, Bubser M, Deutch AY (2012) The effects of nigrostriatal dopamine depletion on the thalamic parafascicular nucleus. Brain Res 1446:46–55. doi:10.1016/j.brainres.2012.01.040
Lai H, Tsumori T, Shiroyama T, Yokota S, Nakano K, Yasui Y (2000) Morphological evidence for a vestibulo-thalamo-striatal pathway via the parafascicular nucleus in the rat. Brain Res 872:208–214
Liedgren SR, Schwarz DW (1976) Vestibular evoked potentials in thalamus and basal ganglia of the squirrel monkey (Saimiri sciureus). Acta Otolaryngol 81:73–82
Liedgren SR, Milne AC, Schwarz DW, Tomlinson RD (1976) Representation of vestibular afferents in somatosensory thalamic nuclei of the squirrel monkey (Saimiri sciureus). J Neurophysiol 39:601–612
Lopez C, Blanke O (2011) The thalamocortical vestibular system in animals and humans. Brain Res Rev 67:119–146. doi:10.1016/j.brainresrev.2010.12.002
Matsuo S, Hosogai M, Nakao S (1994) Ascending projections of posterior canal-activated excitatory and inhibitory secondary vestibular neurons to the mesodiencephalon in cats. Exp Brain Res 100:7–17
Matsuo S, Hosogai M, Matsui H, Ikoma H (1995) Posterior canal-activated vestibulocortical pathways in cats. Neurosci Lett 183:131–134
Matsuo S, Takeuchi H, Ikoma H, Hosogai M (1999) Location of thalamic neurons mediating vestibulo-cortical pathways. Yonago Acta Media 42:163–166
Meng H, Bai RS, Sato H, Imagawa M, Sasaki M, Uchino Y (2001) Otolith-activated vestibulothalamic neurons in cats. Exp Brain Res 141:415–424. doi:10.1007/s00221-001-0902-y
Meng H, May PJ, Dickman JD, Angelaki DE (2007) Vestibular signals in primate thalamus: properties and origins. J Neurosci 27:13590–13602. doi:10.1523/JNEUROSCI.3931-07.2007
Newlands SD, Perachio AA (2003) Central projections of the vestibular nerve: a review and single fiber study in the Mongolian gerbil. Brain Res Bull 60:475–495
Ono S, Kushiro K, Zakir M, Meng H, Sato H, Uchino Y (2000) Properties of utricular and saccular nerve-activated vestibulocerebellar neurons in cats. Exp Brain Res 134:1–8
Pal S, Rosengren SM, Colebatch JG (2009) Stochastic galvanic vestibular stimulation produces a small reduction in sway in Parkinson’s disease. J Vestib Res 19:137–142. doi:10.3233/VES-2009-0360
Park BR, Soh BS, Kim MS, Lee MY, Kim JH, Chun SW (2004) Resolution of the region of secondary somatosensory cortex that responds to stimulation of the horizontal semicircular canals in rats. Neurosci Res Commun 34:174–182
Paxinos G, Watson C (2007) The rat brain in streotaxic coordinates, 6th edn. Academic Press, New York
Pfeiffer C, Serino A, Blanke O (2014) The vestibular system: a spatial reference for bodily self-consciousness. Front Integr Neurosci 8:31. doi:10.3389/fnint.2014.00031
Pisa M (1988) Regional specialization of motor functions in the rat striatum: implications for the treatment of parkinsonism. Prog Neuropsychopharmacol Biol Psychiatry 12:217–224
Raymond J, Dememes D, Marty R (1976) Pathways and ascending vestibular projections emanating from primary nuclei: radioautographic study (author’s transl). Brain Res 111:1–12
Roucoux-Hanus M, Boisacq-Schepens N (1977) Ascending vestibular projections: further results at cortical and thalamic levels in the cat. Exp Brain Res 29:283–292
Samoudi G, Nissbrandt H, Dutia MB, Bergquist F (2012) Noisy galvanic vestibular stimulation promotes GABA release in the substantia nigra and improves locomotion in hemiparkinsonian rats. PLoS One 7:e29308. doi:10.1371/journal.pone.0029308
Sans A, Raymond J, Marty R (1970) Thalamic and cortical responses to electric stimulation of the vestibular nerve in the cat. Exp Brain Res 10:265–275
Shinder ME, Taube JS (2010) Differentiating ascending vestibular pathways to the cortex involved in spatial cognition. J Vestib Res 20:3–23. doi:10.3233/VES-2010-0344
Shiroyama T, Kayahara T, Yasui Y, Nomura J, Nakano K (1995) The vestibular nuclei of the rat project to the lateral part of the thalamic parafascicular nucleus (centromedian nucleus in primates). Brain Res 704:130–134
Shiroyama T, Kayahara T, Yasui Y, Nomura J, Nakano K (1999) Projections of the vestibular nuclei to the thalamus in the rat: a Phaseolus vulgaris leucoagglutinin study. J Comp Neurol 407:318–332
Stiles L, Smith PF (2015) The vestibular-basal ganglia connection: balancing motor control. Brain Res 1597:180–188. doi:10.1016/j.brainres.2014.11.063
Tamlin B, McDonald K, Correll M, Sharpe MH (1993) The immediate effects of vestibular stimulation on gait in patients with Parkinson’s disease. Neurorehabil Neural Repair 7:35–39. doi:10.1177/136140969300700106
Yan W, Zhang QJ, Liu J et al (2008) The neuronal activity of thalamic parafascicular nucleus is conversely regulated by nigrostriatal pathway and pedunculopontine nucleus in the rat. Brain Res 1240:204–212. doi:10.1016/j.brainres.2008.09.015
Acknowledgements
This work was supported by a grant from the Korea Basic Science Institute (D35401).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All experimental procedures were conducted under a protocol approved by the Institutional Animal Care and Use Committee of Wonkwang University and in accordance with WKU12-61.
Electronic supplementary material
Below is the link to the electronic supplementary material.
221_2016_4864_MOESM1_ESM.pdf
Supplementary Fig. 1. Typical changes in single-unit activity in the PFN in response to 30 repetitive stimuli of 5 train pulses for 30 s (a) and continuous 200-Hz stimulation for 30 s (b). Spontaneous firing of PFN neurons was minimally changed by 30 repetitive stimulations of 5 train pulses with a 20-ms interval. In contrast, 200 Hz continuous stimulation caused an increase in the spontaneous firing of PFN neurons (PDF 31 KB)
221_2016_4864_MOESM2_ESM.pdf
Supplementary Fig. 2. Expressions of cFos-IL neurons at different rostrocaudal levels of the PFN of sham-treated animal, which did not receive the stimulation, and in the PFN 90 min following HSC nerve stimulation. The photographs were captured at -4.1-, -4.2-, and − 4.3-mm AP, respectively. The expression of cFos-IL neurons mainly occurred in PFN subregions between AP -4.1 mm and AP -4.2 mm. Ipsi, ipsilateral; Contra, contralateral; fr, fasciculus retroflexus; and Po, posterior thalamic nuclear group. Scale bar = 500 µm (PDF 825 KB)
221_2016_4864_MOESM3_ESM.pdf
Supplementary Fig. 3. Regional distribution of cFos-IL neurons in the contralateral other thalamic nuclei of sham-treated animal and of animal with HSC stimulation. There was a significant increase in cFos-IL neuron expression in the posterior thalamic nuclear group (Po), the ventral posteromedial thalamic nucleus (VPM), and the medial habenular nucleus (MHb) 90 min following HSC nerve stimulation. LPLR, lateral posterior thalamic nucleus, laterorostral; VPL, ventral posterolateral thalamic nucleus; VG1, ventral geniculate nucleus layer 1; fr, fasciculus retroflexus. Scale bar = 500 µm (PDF 154 KB)
Rights and permissions
About this article
Cite this article
Kim, N., Choi, M.A., Koo, H. et al. Activation of the thalamic parafascicular nucleus by electrical stimulation of the peripheral vestibular nerve in rats. Exp Brain Res 235, 1617–1625 (2017). https://doi.org/10.1007/s00221-016-4864-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4864-5