Abstract
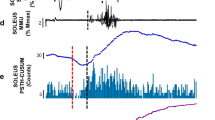
The peristimulus frequencygram (PSF) has recently been shown to illustrate postsynaptic potentials of motoneurones much more reliably than the peristimulus time histogram (PSTH). The aim of this investigation was to examine the profile of the postsynaptic potential (PSP) in soleus motoneurones in response to an H-reflex with and without accompanying M waves of different magnitude by using PSTH and PSF profiles of single motor units. Nine men and five women healthy subjects participated in this study. Electrical stimuli were delivered to the tibial nerve in the popliteal fossa. The reflex response of the soleus muscle was recorded using both surface electromyogram and single motor unit potentials. The PSTH analysis demonstrated that there were four different synaptic events following low-intensity stimulation of the tibial nerve: primary enhancement in firing probability (H-reflex or E1), primary reduction in firing probability (primary silent period or SP1), secondary reduction in firing probability (secondary silent period or SP2), and secondary enhancement in firing probability (E2). On the other hand, the PSF analysis indicated only two reflex responses, long-lasting enhancement in discharge rate including the H-reflex (LLE) and long-lasting decrease in discharge rate (LLD). The results of the two analyses methods are compared and contrasted. While the PSTH demonstrated that there was a silent period (SP1) immediately following the H-reflex, the PSF indicated an increase in discharge rate during the same period. The PSF also indicated that, during SP2 and E2, the discharge rate actually decreased (LLD). It was therefore suggested that LLD involved activation of several inhibitory pathways including the autogenic inhibition of units via the Golgi tendon organs. It was concluded that the PSF could indicate the details of the postsynaptic potentials and is very useful for bringing out previously unknown effects of electrical stimulation of muscle nerves.






Similar content being viewed by others
References
Ashby P, Zilm D (1978) Synaptic connections to individual tibialis anterior motoneurones in man. J Neurol Neurosurg Psychiatr 41:684–689
Awiszus F, Feistner H, Schafer SS (1991) On a method to detect long-latency excitations and inhibitions of single hand muscle motoneurons in man. Exp Brain Res 86:440–446
Brinkworth RS, Türker KS (2003) A method for quantifying reflex responses from intra-muscular and surface electromyogram. J Neurosci Methods 122:179–193
Burne JA, Lippold OC (1996) Reflex inhibition following electrical stimulation over muscle tendons in man. Brain 119:1107–1114
Bussel B, Pierrot Deseilligny E (1977) Inhibition of human motoneurons, probably of Renshaw origin, elicited by an orthodromic motor discharge. J Physiol (London) 269:319–339
Ellaway PH (1978) Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 45:302–303
Garnett R, Stephens JA (1980) The reflex responses of single motor units in human first dorsal interosseous muscle following cutaneous afferent stimulation. J Physiol 303:351–364
Green HJ, Daub B, Houston ME, Thomson JA, Fraser I, Ranney D (1981) Human vastus lateralis and gastrocnemius muscles. A comparative histochemical and biochemical anaysis. J Neurol Sci 52:201–210
Hultborn H (2006) Spinal reflexes, mechanisms and concepts: From Eccles to Lundberg and beyond. Prog Neurobiol 78:215–232
Jami L (1992) Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev 72:623–666
Jankowska E (1992) Interneuronal relay in spinal pathways from proprioceptors. Progr Neurobiol 38:335–378
Kahya MC, Yavuz SU, Türker KS (2010) Cutaneous silent period in human FDI motor units. Exp Brain Res 205:455–463
Khan SI, Burne JA (2009) Afferents contributing to autogenic inhibition of gastrocnemius following electrical stimulation of its tendon. Brain Res 1282:28–37
Knikou M (2008) The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods 171:1–12
Kudina LP, Pantseva RE (1988) Recurrent inhibition of firing motoneurones in man. Electroencephalogr Clin Neurophysiol 69:179–185
Lourenco G, Iglesias C, Cavallari P, Pierrot-Deseilligny E, Marchand-Pauvert V (2006) Mediation of late excitation from human hand muscles via parallel group II spinal and group I transcortical pathways. J Physiol (London) 572:585–603
Pierrot-Deseilligny E, Mazevet D (2000) The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin 30:67–80
Prasartwuth O, Binboğa E, Türker KS (2008) A study of synaptic connection between low threshold afferent fibres in common peroneal nerve and motoneurones in human tibialis anterior. Exp Brain Res 191:465–472
Semmler JG, Türker KS (1994) Compound group I excitatory input is differentially distributed to motoneurones of the human tibialis anterior. Neurosci Lett 178:206–210
Stephens JA, Usherwood TP, Garnett R (1976) Technique for studying synaptic connections of single motoneurones in man. Nature 263:343–344
Türker KS, Cheng HB (1994) Motor-unit firing frequency can be used for the estimation of synaptic potentials in human motoneurons. J Neurosci Methods 53:225–234
Türker KS, Miles TS, Le HT (1988) The lip-clip: a simple, low-impedance ground electrode for use in human electrophysiology. Brain Res Bull 21:139–141
Türker KS, Powers RK (1999) Effects of large excitatory and inhibitory inputs on motoneuron discharge rate and probability. J Neurophysiol 82:829–840
Türker KS, Powers RK (2003) Estimation of postsynaptic potentials in rat hypoglossal motoneurones: insights for human work. J Physiol 551:419–431
Türker KS, Powers RK (2005) Black box revisited: a technique for estimating postsynaptic potentials in neurons. Trends Neurosci 28:379–386
Türker KS, Yang J, Brodin P (1997a) Conditions for excitatory or inhibitory masseteric reflexes elicited by tooth pressure in man. Arch Oral Biol 42:121–128
Türker KS, Yang J, Scutter SD (1997b) Tendon tap induces a single long-lasting excitatory reflex in the motoneurons of human soleus muscle. Exp Brain Res 115:169–173
Ushiba J, Kagamihara Y, Masakado Y (2003) Reassessment of parameters for applying motor-unit triggered stimuli in peri-stimulus time histograms. Brain Res 990:8–19
Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG (1979) Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59:919–957
Van Boxtel A (1979) Selective effects of vibration on monosynaptic and late EMG responses in human soleus muscle after stimulation of the posterior tibial nerve or a tendon tap. J Neurol Neurosurg Psychiatr 42:995–1004
Voss VH (1971) Tabelle der absoluten und relativen Muskelspindelzahlen der menschilichen Skelettmuskulatur. Anat Anz 129:562–572
Acknowledgments
This study is supported by the Marie Curie Chair project (GenderReflex; MEX-CT-2006-040317) and Turkish Scientific and Technological Research Organization (TUBITAK—107S029—SBAG-3556).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Binboğa, E., Prasartwuth, O., Pehlivan, M. et al. Responses of human soleus motor units to low-threshold stimulation of the tibial nerve. Exp Brain Res 213, 73–86 (2011). https://doi.org/10.1007/s00221-011-2779-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2779-8