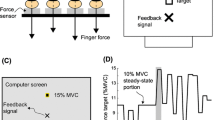
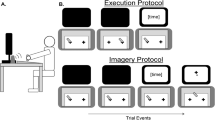
Abstract
Actual and imagined action may be governed by common information and neural processes. This hypothesis has found strong support from a range of chronometric studies showing that it takes the same amount of time to actually move and to imagine moving. However, exceptions have been observed when actual and imagined movements were made under conditions of inertial loading: sometimes the equivalency of actual and imagined movement durations (MDs) has been preserved, and other times it has been disrupted. The purpose of the current study was to test the hypothesis that the appearance and magnitude of actual–imagined MD differences in those studies was dependent on the level of load relative to the maximum loading capacity of the involved effector system [the maximum voluntary load (MVL)]. The experiment required 12 young, healthy humans to actually produce, and to imagine producing, single degree of freedom index finger movements under a range of loads (0, 5, 10, 20, 40, and 80% MVL). As predicted, statistically significant actual–imagined MD differences were absent at lower loads (0–20% MVL), but differences appeared and increased in magnitude with further increases in %MVL (40 and 80% MVL). That pattern of results may relate to the common, everyday experience individuals have in interacting with loads. Participants are likely to have extensive experience interacting with very low loads, but not high loads. It follows that the control of low inertial loads should be governed by complete central representations of action, while representations should be less complete for high loads. A consequence may be increases in the uncertainty of predicting motor output with increases in load. Compensation for the increased uncertainty may appear as increases in the MD values selected during both the preparation and imagery of action—according to a speed-uncertainty trade-off. Then, during actual action, MD may be reduced if movement-related feedback indicates that a faster movement would succeed.



Similar content being viewed by others
Notes
An important contribution of the research by Papaxanthis et al. (2002) and Gentili et al. (2004) was their demonstration that the actual–imagined MD equivalence was preserved as movement dynamics increased in complexity. In Papaxanthis et al. (2002), gravitational torque changed continuously during vertical movements but remained constant during horizontal movements, and in Gentili et al. (2004), there was an increase from one to two degrees of freedom involved in movement. Such results indicate that internal models governing the mental simulation of action contain accurate, detailed knowledge of the dynamics of actual motor output.
It should be noted that judgments of the degree of novelty or effort for moving a particular load may vary according to occupational and cultural background. Bastien et al. (2005) provide a seemingly extreme example: Nepalese porters regularly carry head-supported loads ranging from 100 to 200% of their body weight, at high altitudes and along steep mountain paths.
In the study by Gentili et al. (2004), the 4 kg load used during horizontal shoulder movements might also be at the lower end of the continuum of loads for the action. The arm moved across a horizontal surface on wheels. This would lower the effective load by minimizing the effects of gravity (the inertial resistance) so that after the initial impulse of force the limb could cruise to the target (Gentili et al. 2004, Fig. 1).
The loads used in the current study allowed an examination of AMDs and IMDs over a wide range of loads, but with greater emphasis on a description of change at the lower end of the range (0, 5, 10, 20, 40, and 80% MVL). The choice of those levels of load, within that range, was based on two main considerations: first, including a higher concentration of lower loads might provide greater precision in capturing the point along the load continuum that marked the disruption of the actual–imagined MD equivalency. In the absence of relevant information in the literature, one hypothesis was that the preservation-to-disruption transition might first emerge along the lower portion of the range of loads. Second, having a greater concentration of low loads, randomly intermixed with a few high loads, might dilute a potential build-up of fatigue. In contrast, increasing the number of high loads would increase the effects of fatigue.
Would variation in the amount of practice have influenced the current finding that actual–imagined MD differences increase with increases in %MVL? That question is addressed here, first, through an examination of potential performance changes over series (trials) in the current data, and, second, by examining how differences in the number of trials used among the prior studies on load and imagery may have influenced the presence or absence of actual–imagined MD differences.
To examine potential changes over the four series, the data were submitted to a three-way performance condition (2) by load (6) by series (4) ANOVA, and then separate two-way load (6) by series (4) ANOVAs were applied to the actual and imagined performance conditions. Only the main effects for series and interactions with series were relevant to the current question. All other main effects and interactions from the three- and two-way ANOVAs were significant (P S < 0.05), and those results matched the ANOVA outcomes already reported in the “Results”. The only other significant findings were a small performance condition by series interaction from the three-way ANOVA that appeared to reflect reductions in AMD over series without change in IMD, F 3,33 = 3.01, P = 0.044, and a series effect from the two-way ANOVA that confirmed the reliability of the reductions in AMD, F 3,33 = 4.11, P < 0.05. The other two-way ANOVA verified the absence of change in IMD over series, F 3,33 = 0.25, P = 0.862. Thus, with the reductions in AMD there was a tendency for actual–imagined MD differences to increase over series. A prediction that follows is that actual–imagined MD differences should continue to grow with further increases in the number of trials. That is, additional practice might strengthen, or at least not alter, the main findings of the current study (Fig. 2).
The prior studies on load and imagery examined performance as a function of performance condition, load, and a spatial variable (target distance: Decety et al. 1989; target width: Cerritelli et al. 2000; target direction: Papaxanthis et al. 2002; target direction: Gentili et al. 2004). Among those studies, there was relatively wide variation in the number of trials performed at each unique experimental condition (2 trials: Cerritelli et al. 2000; 10 trials: Decety et al. 1989; 10 trials: Papaxanthis et al. 2002; 12 trials: Gentili et al. 2004). In each case, the data were presented as averages across trials within each unique condition. A comparison of the results of those studies, along with the current results, indicates that the across-study variation in the amount of practice was not related to the presence or absence of actual–imagined MD differences. For example, when load levels were high relative to the maximum capacity of the effector system in Cerritelli et al. (2000), in the current study, and in Decety et al. (1989), the number of trials performed under each unique condition was 2, 4, and 10, respectively. Despite those differences, in each case there were still large and reliable actual–imagined MD differences. Even when experience with each unique condition was quite brief, Cerritelli et al. (2000, see Fig. 1) showed that high load IMDs were clearly elevated above the corresponding AMDs at each of the 10 target width conditions in their cyclical aiming task.
For a given combination of A and W requirements, there is evidence that the amount of MD lengthening to achieve a desired reduction in outcome uncertainty is mainly set before movement execution (Meyer et al. 1988; Jeannerod 1994), while participants view the target display (Slifkin and Grilli 2006).
References
Bachmanov AA, Tordoff MG, Beauchamp GK (2000) Basic characteristics of glutamates and umami sensing in the oral cavity and gut. J Nutr 130:935S–941S
Bastien GJ, Schepens B, Willems PA, Heglund NC (2005) Energetics of load carrying in Nepalese porters. Science 308:1755
Brooks VB (1986) The neural basis of motor control. Oxford University Press, New York
Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi F, Porro CA, Rizzolatti G (2004) Neural circuits involved in the recognition of actions performed by nonconspecifics: an fMRI study. J Cogn Neurosci 16:114–126
Cerritelli B, Maruff P, Wilson P, Currie J (2000) The effect of an external load on the force and timing components of mentally represented actions. Behav Brain Res 108:91–96
Cornsweet TN (1962) The staircase method in psychophysics. Am J Psychol 75:485–491
Coyle EF (2005) Improved muscular efficiency displayed as Tour de France champion matures. J Appl Physiol 98:2191–2196
Decety J (1996a) The neurophysiological basis of motor imagery. Behav Brain Res 77:45–52
Decety J (1996b) Do imagined and executed actions share the same neural substrate? Cogn Brain Res 3:87–93
Decety J, Jeannerod M, Prablanc C (1989) The timing of mentally represented actions. Behav Brain Res 34:35–42
Desmurget M, Grafton S (2000) Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci 4:423–431
Ehrsson HH, Geyer S, Naito E (2003) Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J Neurophysiol 90:3304–3316
Fitts PM (1954) The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47:381–391
Fitts PM, Peterson JR (1964) Information capacity of discrete motor responses. J Exp Psychol 67:103–112
Garner WR (1962) Uncertainty and structure as psychological concepts. Wiley, New York
Gentili R, Cahouet V, Ballay Y, Papaxanthis C (2004) Inertial properties of the arm are accurately predicted during motor imagery. Behav Brain Res 155:231–239
Gescheider GA (1997) Psychophysics: the fundamentals, 3rd edn. Lawrence Erlbaum Associates, Mahwah
Gottlieb GL, Corcos DM, Argawal GC (1989a) Strategies for the control of single voluntary movements with one mechanical degree of freedom. Behav Brain Sci 12:189–250
Gottlieb GL, Corcos DM, Argawal GC (1989b) Organizing principles for single-joint movements: I. a speed-sensitive strategy. J Neurophysiol 62:342–357
Hick WE (1952) On the rate of gain of information. Q J Exp Psychol 4:11–26
Hyman R (1953) Stimulus information as a determinant of reaction time. J Exp Psychol 45:188–196
Jeannerod M (1994) The representing brain: neural correlates of motor intention and imagery. Behav Brain Sci 17:187–245
Jeannerod M (1995) Mental imagery in the motor context. Neuropsychologia 11:1419–1432
Jeannerod M (2001) Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage 14:S103–S109
Jeannerod M, Decety J (1995) Mental motor imagery: a window into the representational stages of action. Curr Biol 5:727–732
Jeannerod M, Frak V (1999) Mental imaging of motor activity in humans. Curr Opin Neurobiol 9:735–739
Johannson RS (1996) Sensory control of dextrous manipulation in humans. In: Wing AM, Haggard P, Flanagan JR (eds) Hand and brain: the neurophysiology and psychology of hand movements. Academic, New York, pp 381–414
Johannson RS, Westling G (1984) Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery surfaces. Exp Brain Res 56:550–564
Johannson RS, Westling G (1988) Coordinated isometric muscle commands adequately and erroneously programmed for the weight during lifting task with precision grip. Exp Brain Res 71:59–71
Khan MA, Garry MI, Franks IM (1999) The effect of target size and inertial load on the control of rapid aiming movements: a test of speed-sensitive and speed-insensitive strategies. Exp Brain Res 124:151–158
Lacquaniti F, Soechting JF, Terzuolo CA (1982) Some factors pertinent to the organization and control of arm movements. Brain Res 252:394–397
Latash L, Li Z-M, Zatsiorsky VM (1998) A principle of error compensation studied within a task of force production by a redundant set of fingers. Exp Brain Res 122:131–138
Meyer DE, Abrams RA, Kornblum S, Wright CE, Smith JEK (1988) Optimality in human motor performance: ideal control of rapid aimed movements. Psychol Rev 89:449–482
Mulder T, Zijlstra S, Zijlstra W, Hochstenbach J (2004) The role of motor imagery in learning a totally novel movement. Exp Brain Res 154:211–217
Papaxanthis C, Schieppati M, Gentili R, Pozzo T (2002) Imagined and actual arm movements have similar durations when performed under different conditions of direction and mass. Exp Brain Res 143:447–452
Philips GT, Tzvetkova EI, Stephane M, Carew TJ (2006) Latent memory for sensitization in Aplysia. Learn Mem 13:224–229
Reed CL (2002) Chronometric comparisons of imagery to action: visualizing versus physically performing springboard dives. Mem Cognit 30:1169–1178
Rosenbaum DA (1991) Human motor control. Academic, New York
Schmidt RA, Zelaznik HN, Hawkins B, Frank JS, Quinn JT (1979) Motor-output variability: a theory for the accuracy of rapid motor acts. Psychol Rev 86:415–451
Schwoebel J, Boronat CB, Coslett HB (2002) The man who executed “imagined” movements: evidence for dissociable components of body schema. Brain Cogn 50:1–16
Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y (1996) The mental representation of hand movements after parietal cortex damage. Science 273:1564–1568
Slifkin AB, Grilli SM (2006) Aiming for the future: prospective action difficulty, prescribed difficulty, and Fitts’ law. Exp Brain Res 174:746–753
Slifkin AB, Newell KM (1999) Noise, information transmission and force variability. J Exp Psychol Hum Percept Perform 25:837–851
Stoll T, Huber E, Seifert B, Michel BA, Stucki G (2000) Maximum isometric strength: normative values and gender-specific relation to age. Clin Rheumatol 19:105–113
Winter DA (1990) Biomechanics and motor control of human movement, 2nd edn. Wiley, New York
Wolpert DM, Flanagan JR (2001) Motor prediction. Curr Biol 11:R729–R732
Wolpert DM, Ghahramani Z (2000) Computational principles of movement science. Nat Neurosci 3:1212–1217
Wolpert DM, Ghahramani Z, Jordan MI (1995) An internal model for sensorimotor integration. Science 269:1880–1882
Yue GH, Cole KJ (1992) Strength increases from the motor program: comparison of training with maximal voluntary and imagined muscle contractions. J Neurophysiol 67:1114–1123
Acknowledgments
Rhiannon L. Hamilton assisted with the collection of data for the current study, and Albert F. Smith provided feedback on an earlier version of this paper. The author is grateful.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slifkin, A.B. High loads induce differences between actual and imagined movement duration. Exp Brain Res 185, 297–307 (2008). https://doi.org/10.1007/s00221-007-1154-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-1154-2