Abstract
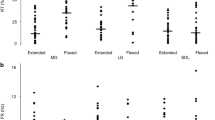
In walking cats, the biarticular medial and lateral gastrocnemius (MG–LG) muscles act to produce extension and flexion torques at the ankle and knee, respectively, and they usually display only one burst of activity beginning just before ground contact and ending near the end of the stance phase. Currently, the MG–LG muscles are considered to function primarily to control extension movements around the ankle joint during the stance phase. However, their flexion action at the knee means that they have the capacity to regulate rotations at the knee, but this role has not yet been clearly defined. Following partial denervation of the other muscles that normally act to flex the knee during swing, we observed that the MG–LG muscles, but not the Soleus muscle (a pure ankle extensor), often generated strong bursts of activity during early swing. These bursts were enhanced following mechanical stimulation of the paw, and they were especially prominent when the leg trailed over an object. They were absent when the leg led over an object. During treadmill walking the swing-related bursts in MG and LG had little influence on ankle flexion at the beginning of swing, but they were associated with slowing of ankle flexion when the leg trailed over an object. We hypothesized that the recruitment of these bursts functions to partially compensate for the reduction in knee torque resulting from the denervation of other knee flexors. Consistent with this hypothesis was our finding that the magnitude of the swing-related activity in the MG–LG muscles was linearly correlated to the extent of the knee flexion and to the peak angular velocity of knee flexion, and that the timing of the bursts was similar to that in the denervated muscles prior to denervation. Our findings suggest that an excitatory pathway exists from the flexor half-center of the central pattern-generating network to MG–LG motoneurons, and that this pathway is strongly regulated by central and/or peripheral signals.









Similar content being viewed by others
References
Abraham LD, Loeb GE (1985) The distal hindlimb musculature of the cat. Patterns of normal use. Exp Brain Res 58:583–593
Angel MJ, Jankowska E, McCrea DA (2005) Candidate interneurones mediating group I disynaptic EPSPs in extensor motoneurones during fictive locomotion in the cat. J Physiol 563:597–610
Bouyer LJD, Rossignol S (2003) Contribution of cutaneous inputs from the hindpaw to the control of locomotion: 2. Spinal cats. J Neurophysiol 90:3640–3653
Buford JA, Smith JL (1993) Adaptive control for backward quadruped walking. III. Stumbling corrective reactions and cutaneous reflex sensitivity. J Neurophysiol 70:1102–1114
Carlson-Kuhta P, Trank TV, Smith JL (1998) Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol 79:1687–1701
Drew T, Jiang W, Widajewicz W (2002) Contribution of the motor cortex to the control of hindlimbs during locomotion in the cat. Brain Res Rev 40:178–191
Engberg I, Lundberg A (1969) An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand 75:614–630
English AW, Weeks OI (1987) An anatomical and functional analysis of cat biceps femoris and semitendinosus muscle. J Morphol 191:161–175
Forssberg H (1979) Stumbling corrective reaction: a phase dependent compensatory reaction during locomotion. J Neurophysiol 42:936–953
Grillner S (1981) Control of locomotion in bipeds, tetrapods and fish. In: Brooks VB (ed) Handbook of physiology, sect 1, vol 2. The nervous system, motor control. American Physiological Society, Bethesda, MD, pp1179–1236
Lavoie S, Drew T (2002) Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with motor cortex. J Neurophysiol 88:1791–1814
Lawrence JH, Nichols TR (1999) A three-dimensional biomechanical analysis of the cat ankle joint complex: II. Effects of ankle joint orientation on evoked isometric joint torque. J Appl Biomech 15:106–119
Matsuyama K, Drew T (2000) Vestibulospinal and reticulospinal activity during locomotion in the intact cat. I. Walking on a level surface. J Neurophysiol 84:2237–2256
McVea D, Tachibana A, Donelan JM, Hulliger M, Pearson KG (2004) Long-term adaptation to persistent environmental perturbations in the walking cat. Neurosci Soc Abstr 34:180.8
Misiaszek JE, Pearson KG (1999) Injecting botulinum toxin into ankle extensors mimics the effects of axotomy. Neurosci Soc Abstr 25:122
O’Donovan MJ, Pinter MJ, Dum RP, Burke RE (1982) Actions of FDL and FHL muscles in intact cats: functional dissociation between anatomical synergists. J Neurophysiol 47:1126–1143
Pearson KG, Fouad K, Misiaszek JE (1999) Adaptive changes in motor activity associated with functional recovery following muscle denervation in walking cats. J Neurophysiol 82:370–381
Perret C (1983) Centrally generated pattern of motoneuron activity during locomotion in the cat. In: Roberts A, Roberts B (eds) Neural origin of rhythmic movements. Cambridge University Press, Cambridge, pp 405–422
Perret C, Cabelguen JM (1980) Main characteristics of the hindlimb locomotor cycle in the decorticate cat with special reference to bifunctional muscles. Brain Res 187:333–352
Quevedo J, Stecina K, Gosgnach S, McCrea DA (2005a) The stumbling corrective reaction during fictive locomotion in the cat. J Neurophysiol 94(3):2045–2052
Quevedo J, Stecina K, McCrea DA (2005b) Intracellular analysis of reflex pathways underlying the stumbling corrective reaction during fictive locomotion in the cat. J Neurophysiol 94(3):2053–2062
Rossignol S (1996) Control of stereotypic limb movements. In: Rowell LB, Shepard JT (eds) Handbook of physiology, sect 12. Exercise: regulation and integration of multiple systems. American Physiological Society, Bethesda, MD, pp 173–216
Smith JL, Betts B, Edgerton VR, Zernicke RF (1980) Rapid ankle extension during paw shakes: selective recruitment of fast ankle extensors. J Neurophysiol 43:612–620
Smith JL, Carlson-Kuhta P, Trank TV (1998) Forms of forward quadrupedal locomotion. III. A comparison of posture, hindlimb kinematics, and motor patterns for downslope and level walking. J Neurophysiol 79:1702–1716
Smith JL, Chung SH, Zernicke RF (1993) Gait-related motor patterns and hindlimb kinetics for the cat trot and gallop. Exp Brain Res 94:308–322
Wand P, Prochazka A, Sontag KH (1980) Neuromuscular responses to gait perturbations in freely moving cats. Exp Brain Res 38:109–114
Widajewicz W, Kably B, Drew T (1994) Motor cortical activity during voluntary gait modifications in the cat. II. Cells related to the hindlimbs. J Neurophysiol 72:2070–2089
Yakovenko S, Mushahwar V, Vanderhorst VGJM, Holstege G, Prochazka A (2002) Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J Neurophysiol 87:1542–1553
Acknowledgements
This work is supported by grants from the Canadian Institutes for Health Research, Alberta Heritage Foundation for Medical Research, and the Natural Science and Engineering Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tachibana, A., McVea, D.A., Donelan, J.M. et al. Recruitment of gastrocnemius muscles during the swing phase of stepping following partial denervation of knee flexor muscles in the cat. Exp Brain Res 169, 449–460 (2006). https://doi.org/10.1007/s00221-005-0160-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0160-5