Abstract
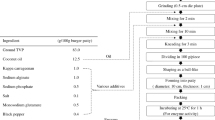
The effect of the addition of Spirulina platensis flour and of extrusion parameters on texture, cooking yield, expressible moisture, total phenolic content (TPC), total flavonoid content (TFC), Trolox equivalent antioxidant activity (TEAC), in vitro protein digestibility (IVPD) and conformational changes of proteins using Fourier-transform infrared spectroscopy (FTIR) of lupin protein based meat analogues was studied. High moisture extrusion (HME) cooking was used to produce the meat analogues. The Spirulina concentration (15, 30 and 50%), extruder barrel temperature (145 °C, 160 °C and 170 °C), water feed (50, 55 and 60%), and screw speed (500, 800 and 1200 rpm) were varied. The Spirulina concentration and extrusion parameters significantly affected physical properties, such as texture, cooking yield and expressible moisture of the extrudates. The addition of Spirulina generally increased the TPC, TFC and TEAC values of the extrudates. Increased temperature and screw speed as well as decreased water feed slightly improved the content of TPC, TFC and TEAC, respectively. The addition of Spirulina at a level of 30% decreased the IVPD of the extrudates from 82 to 75.6%. However, increased water feed and screw speed partly counterbalanced this effect. Protein conformational analyses of the extrudates by FTIR showed that β-sheets were decreased, whereas α-helix, β-turn and antiparallel β-sheets were increased compared to the raw extrusion mixtures. On the whole, the HME process improved the values of TPC, TFC, TEAC and IVPD in the extrudates compared to the raw extrusion mixtures. The addition of Spirulina along with controlled extrusion parameters can deliver meat analogues with improved physico-chemical and nutritional properties.

Similar content being viewed by others
References
FAO (2009) Global agriculture towards 2050, high-level expert forum, how to feed the world 2050, Rome 12–13 October 2009. Food and Agriculture Organization of United Nations FAO
Boland MJ, Rae AN, Vereijken JM, Meuwissen MPM, Fischer ARH, van Boekel MAJS, Rutherfurd SM, Gruppen H, Moughan PJ, Hendriks WH (2013) The future supply of animal-derived protein for human consumption. Trends Food Sci Technol 29:62–73. https://doi.org/10.1016/j.tifs.2012.07.002
Tilman D, Clark M (2014) Global diets link environmental sustainability and human health. Nature 515:518–522. https://doi.org/10.1038/nature13959
Wijffels RH, Barbosa MJ, Eppink MHM (2010) Microalgae for the production of bulk chemicals and biofuels. Biofuel Bioprod Biorefin 4(3):287–295. https://doi.org/10.1002/bbb.215
Milledge JJ (2011) Commercial application of microalgae other than as biofuels: a brief review. Rev Environ Sci Biotechnol 10(31):4131–4141. https://doi.org/10.1007/s11157-010-9214-7
Plaza M, Herrero M, Cifuentes A, Ibáñez E (2009) Innovative natural functional ingredients from microalgae. J Agric Food Chem 57(16):7159–7170. https://doi.org/10.1021/jf901070g
Richmond A (2004) Handbook of microalgal Culture: biotechnology and applied phycology. Blackwell Science Ltd., Hoboken
Sathasivam R, Radhakrishnan R, Hashem A, Abd EF (2017) Microalgae metabolites: a rich source for food and medicine. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2017.11.003
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65(6):635–648. https://doi.org/10.1007/s00253-004-1647-x
Yamaguchi K (1997) Recent advances in microalgal bioscience in Japan, with special reference to utilization of biomass and metabolites: a review. J Appl Phycol 8(6):487–502
Lee YK (2000) Commercial production of microalgae in the Asia-Pacific rim. J Appl Phycol 9(5):403–411
Liang S, Xueming L, Chen F, Chen Z (2004) Current microalgal health food R&D activities in China. Hydrobiol 512:45–48
Romano A, Torrieri E, Masi P, Cavella S (2011) Effects of dietary fiber on structure formation in bread during baking process. J Cereal Sci 49(2):190–201
Wild F (2016) Manufacture of meat analogues through high moisture extrusion. Reference Module in Food Science. Elsevier. https://doi.org/10.1016/b978-0-08-100596-5.03281-1-9
Noguchi A (1990) Extrusion cooking of high moisture protein foods. In: Mercier C, Linko P, Harper JM (eds) Extrusion cooking. AACC, Minnesota, pp 343–369
Bashir S, Sharif MK, Butt MS, Rizvi SSH, Paraman I, Ejaz R (2017) Preparation of micronutrients fortified Spirulina supplemented rice-soy crisps processed through novel supercritical fluid extrusion. J Food Process Preserv. https://doi.org/10.1111/jfpp.12986
Tańska M, Konopka I, Ruszkowska M (2017) Sensory, physico-chemical and water sorption properties of corn extrudates enriched with Spirulina. Plant Foods Hum Nutr. https://doi.org/10.1007/s11130-017-0628-z
Lucas BF, de Morais MG, Santos TD, Costa JAV (2017) Spirulina for snack enrichment: nutritional, physical and sensory evaluations. LWT Food Sci Technol. https://doi.org/10.1016/j.lwt.2017.12.032
Grahl S, Palanisamy M, Strack M, Meier-Dinkel L, Töpfl S, Mörlein D (2018) Towards more sustainable meat alternatives: how technical parameters affect the sensory properties of extrusion products derived from soy and algae. J Clean Prod 198:962–971. https://doi.org/10.1016/j.jclepro.2018.07.041
Trugo LC, von Baer D, von Baer E (2003) Lupin. In: Caballero B (ed) Encyclopedia of food sciences and nutrition, 2nd edn. Academic Press, Cambridge, pp 3623–3629
Palanisamy M, Franke K, Berger RG, Heinz V, Töpfl S (2018) High moisture extrusion of lupin protein: influence of extrusion parameters on extruder responses and product properties. J Sci Food Agric 99:2175–2185. https://doi.org/10.1002/jsfa.9410
Palanisamy M, Töpfl S, Aganovic K, Berger RG (2018) Influence of iota carrageenan addition on the properties of soya protein meat analogues. LWT Food Sci Technol 87:546–552. https://doi.org/10.1016/j.lwt.2017.09.029
Grau R, Hamm R (1957) Über das Wasserbindungsvermoegen im Wasserbindung Wasserbindung im Fleisch. Fleischwirtschaft 32:295
Martínez-Villaluenga C, Zieliński H, Frias J, Piskuła MK, Kozłowska H, Vidal-Valverde C (2009) Antioxidant capacity and polyphenolic content of high-protein lupin products. Food Chem 112:84–88. https://doi.org/10.1016/j.foodchem.2008.05.040
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical. Free Radic Biol Med 26:1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Hsu HW, Vavak DL, Satterlee LD, Miller GA (1977) A multienzyme technique for estimating protein digestibility. J Food Sci 42:1269–1273. https://doi.org/10.1111/j.1365-2621.1977.tb14476.x
Tinus T, Damour M, Van Riel V, Sopade PA (2012) Particle size-starch-protein digestibility relationships in cowpea (Vigna unguiculata). J Food Eng 113:254–264. https://doi.org/10.1016/j.jfoodeng.2012.05.041
Lahlali R, Jiang Y, Kumar S, Karunakaran C, Liu X, Borondics F, Hallin E, Bueckert R (2014) ATR–FTIR spectroscopy reveals involvement of lipids and proteins of intact pea pollen grains to heat stress tolerance. Front Plant Sci 5(747):1–10. https://doi.org/10.3389/fpls.2014.00747
Guo XJ, Wang RQ (2018) Changes in secondary structure of myofibrillar protein and its relationship with water dynamic changes during storage of battered and deep-fried pork slices. Food Sci Biotechnol. https://doi.org/10.1007/s10068-018-0395-0
Fang Y, Zhang B, Wei Y (2014) Effects of the specific mechanical energy on the physicochemical properties of texturized soy protein during high-moisture extrusion cooking. J Food Eng 121:32–38. https://doi.org/10.1016/j.jfoodeng.2013.08.002
Lin S, Huff HE, Hsieh F (2000) Texture and chemical characteristics of soy protein meat analog extruded at high moisture. J Food Sci 65:264–269. https://doi.org/10.1111/j.1365-2621.2000.tb15991.x
Queguiner C, Dumay E, Salou-cavalier C, Cheftel JC (1992) Microcoagulation of a whey protein isolate by extrusion cooking at acid pH. J Food Sci 57(3):610–616
Puyol P, Cotter PF, Mulvihill DM (1999) Thermal gelation of commercial whey protein concentrate: influence of pH 4.6 insoluble protein on thermal gelation. Int J Dairy Technol 52:81–91
Sorgentini DA, Wagner JR, Anon MC (1995) Effects of thermal treatment of soy protein isolate on the characteristics and structure-function relationship of soluble and insoluble fractions. J Agric Food Chem 43:2471–2479. https://doi.org/10.1021/jf00057a029
Vardhanabhuti B, Foegeding EA, McGuffey MK, Daubert CR, Swaisgood HE (2001) Gelation properties of dispersions containing polymerized and native whey protein isolate. Food Hydrocoll 15:165–175. https://doi.org/10.1016/S0268-005X(00)00062-X
El-baky HHA, El-Baz FK, El-baroty GS (2009) Production of phenolic compounds from Spirulina maxima microalgae and its protective effects in vitro toward hepatotoxicity model. EJEAFChe 8(11):7059–7067
Machu L, Misurcova L, Ambrozova JV, Orsavova J, Mlcek J, Sochor J, Jurikova T (2015) Phenolic content and antioxidant capacity in algal food products. Molecules 20:1118–1133. https://doi.org/10.3390/molecules20011118
Batista AP, Niccolai A, Fradinho P, Fragoso S, Bursic I, Rodolfi L, Biondi N, Tredici MR, Sousa I, Raymundo A (2017) Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res 26:161–171. https://doi.org/10.1016/j.algal.2017.07.017
Fradique M, Batista AP, Nunes MC, Gouveia L, Bandarra NM, Raymundo A (2013) Isochrysis galbana and Diacronema vlkianum biomass incorporation in pasta products as PUFA’s source. LWT Food Sci Technol 50:312–319. https://doi.org/10.1016/j.lwt.2012.05.006
Nayak B, Liu RH, Berrios JDJ, Tang J, Derito C (2011) Bioactivity of antioxidants in extruded products prepared from purple potato and dry pea flours. J Agric Food Chem 59:8233–8243. https://doi.org/10.1021/jf200732p
Brennan C, Brennan M, Derbyshire E, Tiwari BK (2011) Effects of extrusion on the polyphenols, vitamins and antioxidant activity of foods. Trends Food Sci Technol 22:570–575. https://doi.org/10.1016/j.tifs.2011.05.007
Gulati P, Weier SA, Santra D, Zhang Y, Rose DJ (2016) Effects of feed moisture and extruder screw speed and temperature on physical characteristics and antioxidant activity of extruded proso millet (Panicum miliaceum) flour. Int J Food Sci Technol 51:114–122. https://doi.org/10.1111/ijfs.12974
Rufián-henares JA, Delgado-andrade C (2009) Effect of digestive process on Maillard reaction indexes and antioxidant properties of breakfast cereals. Food Res Int 42:394–400. https://doi.org/10.1016/j.foodres.2009.01.011
Kosińska-Cagnazzo A, Bocquel D, Marmillod I, Andlauer W (2017) Stability of goji bioactives during extrusion cooking process. Food Chem 230:250–256. https://doi.org/10.1016/j.foodchem.2017.03.035
Wani SA, Kumar P (2018) Influence on the antioxidant, structural and pasting properties of snacks with fenugreek, oats and green pea. J Saudi Soc Agric Sci. https://doi.org/10.1016/j.jssas.2018.01.001
Altan A, Mccarthy KL, Maskan M (2009) Effect of screw configuration and raw material on some properties of barley extrudates. J Food Eng 92:377–382. https://doi.org/10.1016/j.jfoodeng.2008.12.010
White BL, Howard LR, Prior RL (2010) Polyphenolic composition and antioxidant capacity of extruded cranberry pomace. J Agric Food Chem 58:4037–4042. https://doi.org/10.1021/jf902838b
Sharma P, Gujral HS, Singh B (2012) Antioxidant activity of barley as affected by extrusion cooking. Food Chem 131:1406–1413. https://doi.org/10.1016/j.foodchem.2011.10.009
Ruiz-Ruiz J, Martínez-Ayala A, Drago S, González R, Betancur-Ancona D, Chel-Guerrero L (2008) Extrusion of a hard-to-cook bean (Phaseolus vulgaris L.) and quality protein maize (Zea mays L.) flour blend. LWT Food Sci Technol 41:1799–1807. https://doi.org/10.1016/j.lwt.2008.01.005
Kose A, Ozen MO, Elibol M, Oncel SS (2017) Investigation of in vitro digestibility of dietary microalga Chlorella vulgaris and cyanobacterium Spirulina platensis as a nutritional supplement. 3 Biotech 7(170):1–7. https://doi.org/10.1007/s13205-017-0832-4
Zhou L, Yang Y, Ren H, Zhao Y, Wang Z, Wu F, Xiao Z (2016) Structural changes in rice bran protein upon different extrusion temperatures: A raman spectroscopy study. J Chem. https://doi.org/10.1155/2016/6898715
Beck SM, Knoerzer K, Sellahewa J, Emin MA, Arcot J (2017) Effect of different heat-treatment times and applied shear on secondary structure, molecular weight distribution, solubility and rheological properties of pea protein isolate as investigated by capillary rheometry. J Food Eng 208:66–76. https://doi.org/10.1016/j.jfoodeng.2017.03.016
Bai M, Qin G, Sun Z, Long G (2016) Relationship between molecular structure characteristics of feed proteins and protein in vitro digestibility and solubility. Asian Australas J Anim Sci 29:1159–1165. https://doi.org/10.5713/ajas.15.0701
Carbonaro M, Maselli P, Nucara A (2012) Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: a Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 43:911–921. https://doi.org/10.1007/s00726-011-1151-4
Acknowledgements
The authors would like to thank Claus Rüscher, Institut für Mineralogie, Leibniz University Hannover, for helping with the FTIR experiments and data analyses. We also appreciate the assistance of Knut Franke with statistics. This study was supported by the “Niedersächsisches Vorab” programme of the Ministry for Science and Culture of Lower Saxony (Grant # ZN 3041).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Human or animal studies
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Palanisamy, M., Töpfl, S., Berger, R.G. et al. Physico-chemical and nutritional properties of meat analogues based on Spirulina/lupin protein mixtures. Eur Food Res Technol 245, 1889–1898 (2019). https://doi.org/10.1007/s00217-019-03298-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03298-w