Abstract
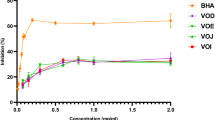
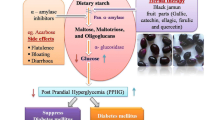
Citrus × clementina juice obtained from fruits collected in three different areas (flood plain, hill and coastal plain) was investigated for the chemical composition, radical scavenging properties (DPPH and ABTS tests), and α-amylase and α-glucosidase inhibitory activity. Neohesperidin (72.96–116.50 mg/100 mL), hesperidin (55.24–69.52 mg/100 mL) and narirutin (7.21–12.13 mg/100 mL) are the main flavonoids identified by HPLC analyses. In carbohydrate hydrolysing enzymes inhibitory activity tests, samples showed higher potency against α-glucosidase. Juice from hill was the most active with an IC50 value of 77.79 μg/mL. Data on the radical scavenging activity revealed the following trend of potency flood plain > coastal plain > hill. These results could help farmers to select fruits for different industrial purpose such as functional food and matrix to extract nutraceutical products.




Similar content being viewed by others
Abbreviations
- ABTS:
-
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)
- ADA:
-
American Diabetes Association
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl
- JNK:
-
C-Jun NH2-terminal kinase
- PCA:
-
Principal component analysis
- ROS:
-
Reactive oxygen species
- T1DM:
-
Type 1 diabetes
- T2DM:
-
Type 2 diabetes
- TNF-α:
-
Tumour-necrosis factor-α
References
Berner L, O’Donnell J (1998) Functional foods and health claims legislation: applications to dairy foods. Int Dairy J 8:355–362
Milind L (2008) Nutritive and medicinal value of Citrus fruit. In: Ladaniya M (ed) Citrus fruit: biology, technology and evaluation. Academic Press, Salt Lake City, pp 501–515 (2038 S 1500E Salt Lake City UT 84105 USA El ). ISBN 8601407120555
Patil BS, Jayaprakasha GK, Murthy KNC, Vikram A (2009) Bioactive compounds: historical perspectives, opportunities, and challenges. J Agric Food Chem 57:8142–8160
Cutuli G, Di Martino E, Lo Giudice V, Pennisi L, Raciti G, Russo F, Scuderi A, Spina P (1985) Trattato di agrumicoltura. Edagricole, Bologna
Alfadda AA, Sallam RM (2012) Reactive Oxygen Species in Health and Disease. J Biomed Biotechnol Article ID 936486
Houstis N, Rosen ED, Lander ES (2006) Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440:944–948
Sivitz WI, Yorek MA (2010) Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal 12:537–577
Tiwari AK, Rao JM (2002) Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci 83:30–38
Tundis R, Loizzo MR, Menichini F (2010) Natural products as α-amylase and α-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini Rev Med Chem 10:315–331
Official methods of analysis, AOAC, Association of Official Analytical Chemists. Edition 18: AOAC (2005)
IFU (3-17-58) International Federation of Fruit Juice Producers. Methods of analysis and microbiological methods. http://www.ifu-fruitjuice.com/ifu-methods
Loizzo MR, Bonesi M, Pugliese A, Menichini F, Tundis R (2014) Chemical composition and bioactivity of dried fruits and honey of Ficus carica cultivars Dottato, San Francesco and Citrullara. J Sci Food Agric 94:2179–2186
Rahal A, Kumar A, Singh A (2014) Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Res Int 761264:1–19
Loizzo MR, Bonesi M, Menichini F, Tenuta MC, Leporini M, Tundis R (2016) Antioxidant and carbohydrate-hydrolysing enzymes potential of Sechium edule (Jacq.) Swartz (Cucurbitaceae) peel, leaves and pulp fresh and processed. Plant Foods Hum Nutr 71:381–387
Sun T, Tanumihardjo SA (2007) An integrated approach to evaluate food antioxidant capacity. J Food Sci 72:159–165
MIRAAF (1994) Metodi Ufficiali di analisi chimica del suolo. Roma, Italy
SISS (1985) Metodi normalizzati analisi del suolo. Edagricole, Bologna
Embleton TM, Jones WW, Labanauskas CH, Reuther W (1973) Leaf analyses as a diagnostic tool and guide to fertilization. In: Reuther W (ed) The Citrus industry. University of California, Berkeley, pp 183–210
White JG, Zasoski RJ (1999) Mapping soil micronutrients. Field Crops Res 60:11–26
Bermejo A, Pardo J, Morales J, Cano A (2016) Comparative study of bioactive components and quality from juices of different mandarins: discriminant multivariate analysis of their primary and secondary metabolites. Agricu Sci 7:341–351
Al-Mouei R, Choumane W (2014) Physiochemical juice characteristics of various Citrus species in Syria. Int J Plant Soil Sci 3:1083–1095
Boudries H, Madani K, Touati N, Souagui S, Medouni S, Chibane M (2012) Pulp antioxidant activities, mineral contents and juice nutritional properties of Algerian Clementine Cultivars and Mandarin. Afr J Biotechnol 11:4258–4267
Sdiri S, Bermejo A, Aleza P, Navarro P, Salvador A (2012) Phenolic composition, organic acids, sugars, vitamin C and antioxidant activity in the juice of two new triploid late-season mandarins. Food Res Int 49:462–468
Xu G, Liu D, Chen J, Ye X, Ma Y, Shi J (2008) Juice components and antioxidant capacity of citrus varieties cultivated in China. Food Chem 106:545–551
Milella L, Caruso M, Galgano F, Favati F, Padula MC, Martelli G (2011) Role of the cultivar in choosing Clementine fruits with a high level of health-promoting compounds. J Agric Food Chem 59:5293–5298
Dhuique-Mayer C, Caris-Veyrat C, Ollitrault P, Curk F, Amiot MJ (2005) Varietal and interspecific influence on micronutrient contents in citrus from the Mediterranean area. J Agric Food Chem 53:2140–2145
Rapisarda P, Bellomo SE, Fabroni S, Russo G (2008) Juice quality of two new mandarin-like hybrids (Citrus clementina Hort. Ex tan × Citrus sinensis L. Osbeck) containing anthocyanins. J Agric Food Chem 56:2074–2078
Rapisarda P, Pannuzzo P, Romano G, Russo G (2003) Juice components of a new pigmented citrus hybrid Citrus sinensis (L.) Osbeck × Citrus clementina Hort. ex Tan. J Agric Food Chem 51:1611–1616
Atmani D, Chaher N, Atmani D, Berboucha M, Debbache N, Boudaoud H (2009) Flavonoids in human health: from structure to biological activity. Curr Nutr Food Sci 5:225–237
Gattuso G, Barreca D, Gargiulli C, Leuzzi U, Caristi C (2007) Flavonoid composition of Citrus juices. Molecules 12:1641–1673
Kanaze FI, Gabrielli C, Kokkalou E, Georgarakis M, Niopas I (2003) Simultaneous reversed-phase high-performance liquid chromatographic method for the determination of diosmin, naringin and hesperidin in different Citrus fruit juices and pharmaceutical formulation. J Pharm Biomed Anal 33:243–249
Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H (2006) Flavonoid composition of fruit tissues of citrus species. Biosci Biotechnol Biochem 70:178–192
Aruoma OI, Landes B, Ramful-Baboolall D, Bourdon E, Neergheen-Bhujun V, Wagner KH, Bahorun T (2012) Functional benefits of citrus fruits in the management of diabetes. Prev Med 54:S12–S16
Abirami A, Nagarani G, Siddhuraju P (2014) In vitro antioxidant, anti-diabetic, cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C. maxima fruits. Food Sci Hum Well 3:16–25
Owira PM, Ojewole JA (2009) Grapefruit juice improves glycaemic control but exacerbates metformin-induced lactic acidosis in non-diabetic rats. Methods Find Exp Clin Pharmacol 31:563–570
Mollace V, Sacco I, Janda E, Malara C, Ventrice D, Colica C, Visalli V, Muscoli S, Ragusa S, Muscoli C, Rotiroti D, Romeo F (2011) Hypolipemic and hypoglycaemic activity of bergamot polyphenols: from animal models to human studies. Fitoterapia 82:309–316
Tadera K, Minami Y, Takamatsu K, Matsuoka T (2010) Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J Nutr Sci Vitaminol 52:149–153
Tundis R, Bonesi M, Sicari V, Pellicanò TM, Tenuta MC, Leporini M, Menichini F, Loizzo MR (2016) Poncirus trifoliata (L.) Raf.: chemical composition, antioxidant properties and hypoglycaemic activity via the inhibition of α-amylase and α-glucosidase enzymes. J Funct Foods 25:477–485
Kim Y, Keogh JB, Clifton PM (2016) Polyphenols and glycemic control. Nutrients 8:17. doi:10.3390/nu8010017
Shen W, Xu Y, Lu YH (2012) Inhibitory effects of citrus flavonoids on starch digestion and antihyperglycemic effects in HepG2 cells. J Agric Food Chem 60:9609–9619
Jia S, Hu Y, Zhang W, Zhao X, Chen Y, Sun C, Li X, Chen K (2015) Hypoglycemic and hypolipidemic effects of neohesperidin derived from Citrus aurantium L. in diabetic KKA(y) mice. Food Funct 6:878–886
Seshagirirao P, Giri KV (1942) The mechanism of β-amylase inhibition by vitamin C. Proc Ind Acad Sci Sec B 16:190–204
Ullah A, Khan A, Khan I (2016) Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm J 24:547–553
Russo D, Bonomo MG, Salzano G, Martelli GBG, Milella L (2012) Nutraceutical properties of Citrus clementina juices. Pharmacologyonline 1:84–93
Codoñer-Franch P, López-Jaén AB, Muñiz P, Sentandreu E, Bellés VV (2008) Mandarin juice improves the antioxidant status of hypercholesterolemic children. J Pediatr Gastroenterol Nutr 47:349–355
Acknowledgements
The authors are grateful to Falco farms for supplying the samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was not specifically funded.
Conflict of interest
The authors declare that there are no conflicts of interest.
Compliance with ethics requirements
Research does not involve any human participants and/or animal.
Rights and permissions
About this article
Cite this article
Loizzo, M.R., Leporini, M., Sicari, V. et al. Investigating the in vitro hypoglycaemic and antioxidant properties of Citrus × clementina Hort. juice. Eur Food Res Technol 244, 523–534 (2018). https://doi.org/10.1007/s00217-017-2978-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-2978-z