Abstract
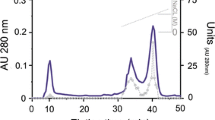
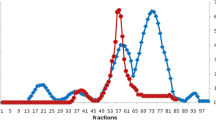
Pepsins 1 and 2 from the stomach of skipjack tuna (Katsuwonus pelamis) were purified to homogeneity by using a series of chromatographic purification involving DEAE-cellulose, Sephadex G-50 and Sephadex G-75 with increase in purity of 246-fold and 213-fold, respectively. Molecular weights of pepsins 1 and 2 were estimated by SDS–PAGE to be 33.9 and 33.7 kDa, respectively. The N-terminal amino acid sequences of the first 20 amino acids of both isoenzymes were YQDGTEPMTNDADLSYYGVI. The optimal pH and temperature for pepsin 1 were 2.5 and 50 °C, respectively, while pepsin 2 showed optimal activity at pH 2.0 and 45 °C. The activity of two pepsins was stable in the pH range of 2–5 and at temperatures up to 50 °C. The activity of purified pepsins was strongly inhibited by pepstatin A in a dose-dependent manner. SDS and cysteine showed inhibitory effects toward both pepsins. Activity of pepsin 2 was slightly activated by NaCl, but NaCl had no effect on pepsin 1. Pepsins 1 and 2 had high affinity and hydrolytic activity toward hemoglobin with K m of 54 and 71 μM, respectively. k cat of pepsins 1 and 2 were 38.1 and 44.3 s−1, respectively. Both pepsins effectively hydrolyzed bovine serum albumin, egg white, natural actomyosin from brownstripe red snapper muscle and acid-solubilized collagen from arabesque greenling skin. Nevertheless, the hydrolytic activity was slightly less than that of pepsin from porcine stomach.






Similar content being viewed by others
References
Gildberg A (1988) Comp Biochem Physiol B 91:425–435
Kageyama T (2002) Cell Mol Life Sci 59:288–306
Nalinanon S, Benjakul S, Kishimura H (2010) Food Chem 121:49–55
Tanji M, Yakabe E, Kageyama T, Yokobori S-i, Ichinose M, Miki K, Ito H, Takahashi K (2007) Comp Biochem Physiol B 146:412–420
Whitaker JR (1994) Principles of enzymology for the food sciences. Marcel Dekker, New York
Arunchalam K, Haard NF (1985) Comp Biochem Physiol B 80:467–473
Tanji M, Kageyama T, Takahashi K (1988) Eur J Biochem 177:251–259
Gildberg A, Olsen RL, Bjarnason JB (1990) Comp Biochem Physiol B 96:323–330
Zhou Q, Fu XP, Zhang LJ, Su WJ, Cao MJ (2007) Food Chem 103:795–801
Klomklao S, Kishimura H, Yabe M, Benjakul S (2007) Comp Biochem Physiol B 147:682–689
Bougatef A, Balti R, Ben Zaied S, Souissi N, Nasri M (2008) Food Chem 107:777–784
Zhou Q, Liu G-M, Huang Y-Y, Weng L, Hara K, Su W-J, Cao M-J (2008) J Agric Food Chem 56:5401–5406
Wu T, Sun L-C, Du C-H, Cai Q-F, Zhang Q-B, Su W-J, Cao M-J (2009) Food Chem 115:137–142
DFT (2009) Canned tuna situation (HS 16041410001), Department of Foreign Trade, Ministry of Commerce, Thailand. http://www.dft.moc.go.th. Accessed 17 Dec 2009
Klomklao S, Benjakul S, Visessanguan W (2004) J Food Biochem 28:355–372
Nalinanon S, Benjakul S, Visessanguan W, Kishimura H (2008) J Food Sci 73:C413–C419
Nalinanon S, Benjakul S, Visessanguan W, Kishimura H (2009) Process Biochem 44:471–476
Pavlisko A, Vecchi SD, Coppes Z (1999) J Food Biochem 23:451–467
Nalinanon S, Benjakul S, Visessanguan W, Kishimura H (2007) Food Chem 104:593–601
Nalinanon S, Benjakul S, Visessanguan W, Kishimura H (2008) Food Hydrocoll 22:615–622
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) J Biol Chem 193:265–275
Laemmli UK (1970) Nature 227:680–685
Lineweaver H, Burk D (1934) J Am Chem Soc 56:658–666
Benjakul S, Seymour TA, Morrissey MT, An H (1997) J Food Sci 62:729–733
Gildberg A, Raa J (1983) Comp Biochem Physiol A 75:337–342
Xu RA, Wong RJ, Rogers ML, Fletcher GC (1996) J Food Biochem 20:31–48
Karlsen S, Hough E, Olsen RL (1998) Acta Crystallogr D 54:32–46
Hartsuck JA, Koelsch G, Remington SJ (1992) Protein Struct Funct Genet 13:1–25
Narita Y, Oda SI, Moriyama A, Kageyama T (2002) Arch Biochem Biophys 404:177–185
Yakabe E, Tanji M, Ichinose M, Goto S, Miki K, Kurokawa K, Ito H, Kageyama T, Takahashi K (1991) J Biol Chem 266:22436–22443
Kubota M, Ohnuma A (1970) Bull Jap Soc Sci Fish 36:1152–1156
Noda M, Murakami K (1981) Biochim Biophys Acta 658:27–34
Squires EJ, Haard NF, Feltham LAW (1986) Biochem Cell Biol 64:205–214
Castillo-Yañez FJ, Pacheco-Aguilar R, García-Carreño FL, Toro MdlAN-D (2004) Food Chem 85:343–350
Guerard F, Le Gal Y (1987) Comp Biochem Physiol B 88:823–827
Fusek M, Větvička V (1995) Aspartic proteinases: Physiology and pathology. CRC Press, New York
Haard NF (1994) In: Shahidi F, Botta JR (eds) Seafoods: Chemistry. processing technology and quality, Chapman & Hall, UK
Pillai S, Zull JE (1985) J Biol Chem 260:8384–8389
Sánchez-Chiang L, Cisternas E, Ponce O (1987) Comp Biochem Physiol B 87:793–797
Acknowledgments
This work was supported by the Thailand Research Fund under the Royal Golden Jubilee Ph.D. Program to Sitthipong Nalinanon (PHD/0171/2549) and TRF Senior Research Scholar program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nalinanon, S., Benjakul, S. & Kishimura, H. Purification and biochemical properties of pepsins from the stomach of skipjack tuna (Katsuwonus pelamis). Eur Food Res Technol 231, 259–269 (2010). https://doi.org/10.1007/s00217-010-1275-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1275-x