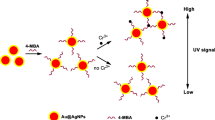
Abstract
Hexavalent chromium is a highly toxic substance, which will pose a serious threat to human life and health and the entire ecosystem. Therefore, it is crucial to establish a simple and rapid detection method for hexavalent chromium. In this work, we fabricated bovine serum albumin–stabilized silver nanocluster (BSA-Ag13 NC) which exhibited photoresponsive oxidase-like activity, catalyzing the oxidation of colorless 3,3′,5,5′-tetramethylbenzidine (TMB) to the blue oxidized state TMB (oxTMB) in a short time. Interestingly, 8-hydroxyquinoline (8-HQ) can significantly inhibit the color reaction of TMB oxidation while Cr(VI) can interact specifically with 8-HQ to restore this chromogenic reaction. Based on the above facts, a colorimetric sensing system for detecting Cr(VI) was developed. The sensing system shows a wide linear range, and good selectivity, with a low detection limit of 2.32 nM. Moreover, this sensing system could be successfully applied to the detection of Cr(VI) in lake water, tap water, and sewage with satisfactory results.






Similar content being viewed by others
References
Li M, Zhou S. α-Fe2O3/polyaniline nanocomposites as an effective catalyst for improving the electrochemical performance of microbial fuel cell. Chem Eng J. 2018;339:539–46. https://doi.org/10.1016/j.cej.2018.02.002.
Xue Q, Li X, Peng Y, Liu P, Peng H, Niu X. Polyethylenimine-stabilized silver nanoclusters act as an oxidoreductase mimic for colorimetric determination of chromium(VI). Microchim Acta. 2020;187(5):263. https://doi.org/10.1007/s00604-020-04232-8.
Wang X, Hu J, Yao B, Wei H, Zhang C, Zhou J, Liu J, Yang S. Bridging environmental and biological monitoring: constructing platform for hexavalent chromium detection and cancer-cells screening based on red fluorescent carbonized polymer dots. Chem Eng J. 2023;451:138524. https://doi.org/10.1016/j.cej.2022.138524.
Korshoj LE, Zaitouna AJ, Lai RY. Methylene blue-mediated electrocatalytic detection of hexavalent chromium. Anal Chem. 2015;87(5):2560–4. https://doi.org/10.1021/acs.analchem.5b00197.
Hsu LC, Wang SL, Tzou YM, Lin CF, Chen JH. The removal and recovery of Cr(VI) by Li/Al layered double hydroxide (LDH). J Hazard Mater. 2007;142(1):242–9. https://doi.org/10.1016/j.jhazmat.2006.08.024.
Yuan H, Zhou H, Zhao Y, Tan H, Antoine R, Zhang S. Metal-organic frameworks encapsulated Ag nanoparticle-nanoclusters with enhanced luminescence for simultaneous detection and removal of chromium(VI). Microchem J. 2022;181:107722. https://doi.org/10.1016/j.microc.2022.107722.
Fierro S, Watanabe T, Akai K, Einaga Y. Highly sensitive detection of Cr6+ on boron doped diamond electrodes. Electrochim Acta. 2012;82:9–11. https://doi.org/10.1016/j.electacta.2012.03.030.
Alula MT, Madingwane ML. Colorimetric quantification of chromium (VI) ions based on oxidoreductase-like activity of Fe3O4. Sens Actuators B. 2020;324:128726. https://doi.org/10.1016/j.snb.2020.128726.
Ullah N, Mansha M, Khan I, Qurashi A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: recent advances and challenges. TrAC Trends Anal Chem. 2018;100:155–66. https://doi.org/10.1016/j.trac.2018.01.002.
Sheikhmohammadi A, Mohseni SM, khodadadi R, Sardar M, Abtahi M, Mahdavi S, Keramati H, Dahaghin Z, Rezaei S, Almasian M, Sarkhosh M, Faraji M, Nazari S. Application of graphene oxide modified with 8-hydroxyquinoline for the adsorption of Cr (VI) from wastewater: optimization, kinetic, thermodynamic and equilibrium studies. J Mol Liq. 2017;233:75–88. https://doi.org/10.1016/j.molliq.2017.02.101.
Mao Y, Gao S, Yao L, Wang L, Qu H, Wu Y, Chen Y, Zheng L. Single-atom nanozyme enabled fast and highly sensitive colorimetric detection of Cr(VI). J Hazard Mater. 2021;408:124898. https://doi.org/10.1016/j.jhazmat.2020.124898.
Frisbie SH, Mitchell EJ, Sarkar B. World Health Organization increases its drinking-water guideline for uranium. Environ Sci Processes Impacts. 2013;15(10):1817–23. https://doi.org/10.1039/C3EM00381G.
Unceta N, Astorkia M, Abrego Z, Gómez-Caballero A, Goicolea MA, Barrio RJ. A novel strategy for Cr(III) and Cr(VI) analysis in dietary supplements by speciated isotope dilution mass spectrometry. Talanta. 2016;154:255–62. https://doi.org/10.1016/j.talanta.2016.03.079.
Motaghedifard M H, Pourmortazavi S M, Mirsadeghi S. Selective and sensitive detection of Cr(VI) pollution in waste water via polyaniline/sulfated zirconium dioxide/multi walled carbon nanotubes nanocomposite based electrochemical sensor. Sens Actuators B. 2021;327:128882. https://doi.org/10.1016/j.snb.2020.128882.
Prabhakaran DC, Ramamurthy PC, Sivry Y, Subramanian S. Electrochemical detection of Cr(VI) and Cr(III) ions present in aqueous solutions using bio-modified carbon paste electrode: a voltammetric study. Int J Environ Anal Chem. 2020;1–21. https://doi.org/10.1080/03067319.2020.1748610.
Mädler S, Todd A, “Skip” Kingston H M, Pamuku M, Sun F, Tat C, Tooley R J, Switzer T A, Furdui V I. Ultra-trace level speciated isotope dilution measurement of Cr(VI) using ion chromatography tandem mass spectrometry in environmental waters. Talanta. 2016;156–157:104–11. https://doi.org/10.1016/j.talanta.2016.04.064.
Miyake Y, Tokumura M, Iwazaki Y, Wang Q, Amagai T, Horii Y, Otsuka H, Tanikawa N, Kobayashi T, Oguchi M. Determination of hexavalent chromium concentration in industrial waste incinerator stack gas by using a modified ion chromatography with post-column derivatization method. J Chromatogr A. 2017;1502:24–9. https://doi.org/10.1016/j.chroma.2017.04.046.
Qi Y, Ma J, Xiu F-R, Gao X. Determination of Cr(VI) based on the peroxidase mimetic catalytic activity of citrate-capped gold nanoparticles. Microchim Acta. 2021;188(8):273. https://doi.org/10.1007/s00604-021-04942-7.
Yilmaz E, Soylak M. Ultrasound assisted-deep eutectic solvent based on emulsification liquid phase microextraction combined with microsample injection flame atomic absorption spectrometry for valence speciation of chromium(III/VI) in environmental samples. Talanta. 2016;160:680–5. https://doi.org/10.1016/j.talanta.2016.08.001.
Zhang X, Liu W, Li X, Zhang Z, Shan D, Xia H, Zhang S, Lu X. Ultrahigh selective colorimetric quantification of chromium(VI) ions based on gold amalgam catalyst oxidoreductase-like activity in water. Anal Chem. 2018;90(24):14309–15. https://doi.org/10.1021/acs.analchem.8b03597.
Lin C-Y, Yu C-J, Lin Y-H, Tseng W-L. Colorimetric sensing of silver(I) and mercury(II) ions based on an assembly of Tween 20-stabilized gold nanoparticles. Anal Chem. 2010;82(16):6830–7. https://doi.org/10.1021/ac1007909.
Chen G, Hai J, Wang H, Liu W, Chen F, Wang B. Gold nanoparticles and the corresponding filter membrane as chemosensors and adsorbents for dual signal amplification detection and fast removal of mercury(II). Nanoscale. 2017;9(9):3315–21. https://doi.org/10.1039/C6NR09638G.
Zhi L, Zeng X, Wang H, Hai J, Yang X, Wang B, Zhu Y. Photocatalysis-based nanoprobes using noble metal–semiconductor heterostructure for visible light-driven in vivo detection of mercury. Anal Chem. 2017;89(14):7649–58. https://doi.org/10.1021/acs.analchem.7b01602.
Zhou X-H, Kong D-M, Shen H-X. Ag+ and cysteine quantitation based on g-quadruplex−hemin DNAzymes disruption by Ag+. Anal Chem. 2010;82(3):789–93. https://doi.org/10.1021/ac902421u.
Gao Z, Deng K, Wang X-D, Miró M, Tang D. High-resolution colorimetric assay for rapid visual readout of phosphatase activity based on gold/silver core/shell nanorod. ACS Appl Mater Interfaces. 2014;6(20):18243–50. https://doi.org/10.1021/am505342r.
Zhuang Y-T, Chen S, Jiang R, Yu Y-L, Wang J-H. Ultrasensitive colorimetric chromium chemosensor based on dye color switching under the Cr(VI)-stimulated Au NPs catalytic activity. Anal Chem. 2019;91(8):5346–53. https://doi.org/10.1021/acs.analchem.9b00339.
Ghayyem S, Swaidan A, Barras A, Dolci M, Faridbod F, Szunerits S, Boukherroub R. Colorimetric detection of chromium (VI) ion using poly(N-phenylglycine) nanoparticles acting as a peroxidase mimetic catalyst. Talanta. 2021;226:122082. https://doi.org/10.1016/j.talanta.2021.122082.
Gao Y, Li X, Li Y, Li T, Zhao Y, Wu A. A simple visual and highly selective colorimetric detection of Hg2+ based on gold nanoparticles modified by 8-hydroxyquinolines and oxalates. Chem Commun. 2014;50(49):6447–50. https://doi.org/10.1039/C4CC00069B.
Lin Y, Ren J, Qu X. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc Chem Res. 2014;47(4):1097–105. https://doi.org/10.1021/ar400250z.
Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y, Qin L, Wei H. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev. 2019;48(4):1004–76. https://doi.org/10.1039/C8CS00457A.
Li Z, Liu W, Ni P, Zhang C, Wang B, Duan G, Chen C, Jiang Y, Lu Y. Carbon dots confined in N-doped carbon as peroxidase-like nanozyme for detection of gastric cancer relevant D-amino acids. Chem Eng J. 2022;428:131396. https://doi.org/10.1016/j.cej.2021.131396.
Fan K, Xi J, Fan L, Wang P, Zhu C, Tang Y, Xu X, Liang M, Jiang B, Yan X, Gao L. In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy. Nat Commun. 2018;9(1):1440. https://doi.org/10.1038/s41467-018-03903-8.
Liu W, Chu L, Zhang C, Ni P, Jiang Y, Wang B, Lu Y, Chen C. Hemin-assisted synthesis of peroxidase-like Fe-N-C nanozymes for detection of ascorbic acid-generating bio-enzymes. Chem Eng J. 2021;415:128876. https://doi.org/10.1016/j.cej.2021.128876.
Li Z, Liu F, Jiang Y, Ni P, Zhang C, Wang B, Chen C, Lu Y. Single-atom Pd catalysts as oxidase mimics with maximum atom utilization for colorimetric analysis. Nano Res. 2022;15(5):4411–20. https://doi.org/10.1007/s12274-021-4029-0.
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577–83. https://doi.org/10.1038/nnano.2007.260.
Sun H, Zhou Y, Ren J, Qu X. Carbon nanozymes: enzymatic properties, catalytic mechanism, and applications. Angew Chem Int Ed. 2018;57(30):9224–37. https://doi.org/10.1002/anie.201712469.
Zhang X, Li G, Wu D, Li X, Hu N, Chen J, Chen G, Wu Y. Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy. Biosens Bioelectron. 2019;137:178–98. https://doi.org/10.1016/j.bios.2019.04.061.
Hu SS, Lin YX, Teng J, Wong W L, Qiu B. In situ deposition of MOF-74(Cu) nanosheet arrays onto carbon cloth to fabricate a sensitive and selective electrocatalytic biosensor and its application for the determination of glucose in human serum. Microchim Acta. 2020;187(12). https://doi.org/10.1007/s00604-020-04634-8.
Shan JY, Yang KL, Xiu WJ, Qiu Q, Dai SL, Yuwen LH, Weng LX, Teng ZG, Wang LH. Cu(2)MoS(4)nanozyme with NIR-II light enhanced catalytic activity for efficient eradication of multidrug-resistant bacteria. SMALL. 2020;16(40). https://doi.org/10.1002/smll.202001099.
Li F, Hu YT, Zhao AQ, Xi YC, Li ZM, He JB. beta-Cyclodextrin coated porous Pd@Au nanostructures with enhanced peroxidase-like activity for colorimetric and paper-based determination of glucose. Microchim Acta. 2020;187(8). https://doi.org/10.1007/s00604-020-04410-8.
Yao L, Kong FY, Wang ZX, Li HY, Zhang R, Fang HL, Wang W. UV-assisted one-pot synthesis of bimetallic Ag-Pt decorated reduced graphene oxide for colorimetric determination of hydrogen peroxide. Microchim Acta. 2020;187(7). https://doi.org/10.1007/s00604-020-04350-3.
Ni P, Liu S, Wang B, Chen C, Jiang Y, Zhang C, Chen J, Lu Y. Light-responsive Au nanoclusters with oxidase-like activity for fluorescent detection of total antioxidant capacity. J Hazard Mater. 2021;411:125106. https://doi.org/10.1016/j.jhazmat.2021.125106.
Ni P, Chen C, Jiang Y, Zhang C, Wang B, Cao B, Li C, Lu Y. Gold nanoclusters-based dual-channel assay for colorimetric and turn-on fluorescent sensing of alkaline phosphatase. Sens Actuators B. 2019;301:127080. https://doi.org/10.1016/j.snb.2019.127080.
Zhang J, Liu J. Light-activated nanozymes: catalytic mechanisms and applications. Nanoscale. 2020;12(5):2914–23. https://doi.org/10.1039/C9NR10822J.
Wu S, Zhang J, Wu P. Photo-modulated nanozymes for biosensing and biomedical applications. Anal Methods. 2019;11(40):5081–8. https://doi.org/10.1039/C9AY01493D.
Wang T, Bai Q, Zhu Z, Xiao H, Jiang F, Du F, Yu WW, Liu M, Sui N. Graphdiyne-supported palladium-iron nanosheets: a dual-functional peroxidase mimetic nanozyme for glutathione detection and antibacterial application. Chem Eng J. 2020;127537. https://doi.org/10.1016/j.cej.2020.127537.
Taylor MG, Mpourmpakis G. Thermodynamic stability of ligand-protected metal nanoclusters. Nat Commun. 2017;8(1):15988. https://doi.org/10.1038/ncomms15988.
Sheng Y, Yang H, Wang Y, Han L, Zhao Y, Fan A. Silver nanoclusters-catalyzed luminol chemiluminescence for hydrogen peroxide and uric acid detection. Talanta. 2017;166:268–74. https://doi.org/10.1016/j.talanta.2017.01.066.
Yu Y, Geng J, Ong EYX, Chellappan V, Tan YN. Bovine serum albulmin protein-templated silver nanocluster (BSA-Ag13): an effective singlet oxygen generator for photodynamic cancer therapy. Adv Healthcare Mater. 2016;5(19):2528–35. https://doi.org/10.1002/adhm.201600312.
Chen CX, Liu WD, Ni PJ, Jiang YY, Zhang CH, Wang B, Li JK, Cao BQ, Lu YZ, Chen W. Engineering two-dimensional Pd nanoplates with exposed highly active 100 facets toward colorimetric acid phosphatase detection. ACS Appl Mater Interfaces. 2019;11(50):47564–70. https://doi.org/10.1021/acsami.9b16279.
Nghia NN, Huy BT, Lee Y-I. Colorimetric detection of chromium(VI) using graphene oxide nanoparticles acting as a peroxidase mimetic catalyst and 8-hydroxyquinoline as an inhibitor. Microchim Acta. 2018;186(1):36. https://doi.org/10.1007/s00604-018-3169-8.
Funding
This work was supported by the National Natural Science Foundation of China (22172063), the Young Taishan Scholar Program (tsqn201812080), the Natural Science Foundation of Shandong Province (ZR2019YQ10), and the Independent Cultivation Program of Innovation Team of Ji’nan City (2021GXRC052).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, Z., Li, Z., Zhao, X. et al. BSA-stabilized silver nanoclusters for efficient photoresponsive colorimetric detection of chromium(VI). Anal Bioanal Chem 415, 1477–1485 (2023). https://doi.org/10.1007/s00216-023-04535-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04535-8