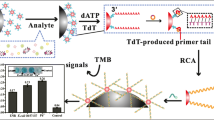
Abstract
A novel sensitive, competitive, and time-resolved luminescence sensor for the detection of ofloxacin (OFL) was developed in this study. The sensor used OFL-specific aptamer as a recognition molecule and rolling circle amplification (RCA) as a signal amplification tool. In this way, the time-resolved luminescence can not only avoid background noise from sample, but also provide robust luminescence for detection. Besides, the separation and enrichment of target veterinary drug can be conducted assisted by magnetic treatment. Under optimal conditions, the logarithmic correlation between the concentration of OFL and the luminescence intensity was found to be linear in the range of 5 × 10−11–5 × 10−8 mol L−1 (R2 = 0.9988), with a detection limit (LOD) of 32.1 pmol L−1. Furthermore, this method was applied to the determination of OFL in chicken and pork samples, exhibiting good recovery (72.5–100%) and repeatability (RSD < 10.0%). These results confirm that this novel established method has good application potential for the detection of OFL in food samples.





Similar content being viewed by others
References
Zhang Y, You Y, Xia Z, Han X, Tian Y, Zhou N. Graphene oxide-based selection and identification of ofloxacin-specific single-stranded DNA aptamers. RSC Adv. 2016;6(101):99540–5.
Monk JP, Campoli-Richards DM. Ofloxacin. Drugs. 1987;33(4):346–91.
Qin X, Geng L, Wang Q, Wang Y. Photoelectrochemical aptasensing of ofloxacin based on the use of a TiO2 nanotube array co-sensitized with a nanocomposite prepared from polydopamine and Ag2S nanoparticles. Microchim Acta. 2019;186(7):430.
Angulo FJ, Collignon P, Powers JH, Chiller TM, Aidara-Kane A, Aarestrup FM. World Health Organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies for the use of antimicrobials in food production animals. Clin Infect Dis. 2009;49(1):132–41.
Commission E. Council Regulation (EEC) No. 2377/90 of 26 June 1990: laying down a community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin. Off J Eur Communities. 1990;224:1–8.
Foundation JFCR. Maximum residue limits (MRLs) list of agricultural chemicals in foods. Tokyo: JFCRF; 2015.
Wang H, Li L, Xing R, Zhang Y, Wu T, Chen B, et al. Screening of antimicrobials in animal-derived foods with desorption corona beam ionization (DCBI) mass spectrometry. Food Chem. 2019;272:411–7.
Li J, Ren X, Diao Y, Chen Y, Wang Q, Jin W, et al. Multiclass analysis of 25 veterinary drugs in milk by ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2018;257:259–64.
Tochi BN, Peng J, Song S, Liu L, Kuang H, Xu C. Production and application of a monoclonal antibody (mAb) against ofloxacin in milk, chicken and pork. Food Agric Immunol. 2016;27(5):643–56.
Zhou X, Wang L, Shen G, et al. Colorimetric determination of ofloxacin using unmodified aptamers and the aggregation of gold nanoparticles[J]. Microchim Acta. 2018;185(7):355.
Adhikari S, Kim D-H. Synthesis of Bi2S3/Bi2WO6 hierarchical microstructures for enhanced visible light driven photocatalytic degradation and photoelectrochemical sensing of ofloxacin. Chem Eng J. 2018;354:692–705.
Vakh C, Alaboud M, Lebedinets S, Bulatov A. A rotating cotton-based disk packed with a cation-exchange resin: separation of ofloxacin from biological fluids followed by chemiluminescence determination. Talanta. 2019;196:117–23.
Reinemann C, von Fritsch UF, Rudolph S, Strehlitz B. Generation and characterization of quinolone-specific DNA aptamers suitable for water monitoring. Biosens Bioelectron. 2016;77:1039–47.
Pilehvar S, Reinemann C, Bottari F, Vanderleyden E, Van Vlierberghe S, Blust R, et al. A joint action of aptamers and gold nanoparticles chemically trapped on a glassy carbon support for the electrochemical sensing of ofloxacin. Sensors Actuators B Chem. 2017;240:1024–35.
Yao CZ, Tong YX. Lanthanide ion-based luminescent nanomaterials for bioimaging. Trac-trend Anal Chem. 2012;39:60–71.
Ni F, Zhu ZC, Tong X, Xie MJ, Zhao Q, Zhong C, et al. Organic emitter integrating aggregation-induced delayed fluorescence and room-temperature phosphorescence characteristics, and its application in time-resolved luminescence imaging. Chem Sci. 2018;9(28):6150–5.
Liu YS, Tu DT, Zhu HM, Chen XY. Lanthanide-doped luminescent nanoprobes: controlled synthesis, optical spectroscopy, and bioapplications. Chem Soc Rev. 2013;42(16):6924–58.
Tang YW, Li M, Gao X, Liu XY, Gao JW, Ma T, et al. A NIR-responsive up-conversion nanoparticle probe of the NaYF4: Er,Yb type and coated with a molecularly imprinted polymer for fluorometric determination of enrofloxacin. Microchim Acta. 2017;184(9):3469–75.
Giri B, Pandey B, Neupane B, Ligler FS. Signal amplification strategies for microfluidic immunoassays. Trac-trend Anal Chem. 2016;79:326–34.
Fire A, Xu S-Q. Rolling replication of short DNA circles. Proc Natl Acad Sci. 1995;92(10):4641–5.
Fan T, Mao Y, Liu F, Zhang W, Lin J-S, Yin J, et al. Label-free fluorescence detection of circulating microRNAs based on duplex-specific nuclease-assisted target recycling coupled with rolling circle amplification. Talanta. 2019;200:480–6.
Gu LD, Yan WL, Liu L, Wang SJ, Zhang X, Lyu MS. Research progress on rolling circle amplification (RCA)-based biomedical sensing. Pharmaceuticals. 2018;11(2):19.
Deng HM, Gao ZQ. Bioanalytical applications of isothermal nucleic acid amplification techniques. Anal Chim Acta. 2015;853:30–45.
Liu X, Zhang X. Aptamer-based technology for food analysis. Appl Biochem Biotechnol. 2015;175(1):603–24.
Zhao L, Huang Y, Dong Y, Han X, Wang S, Liang X. Aptamers and aptasensors for highly specific recognition and sensitive detection of marine biotoxins: recent advances and perspectives. Toxins (Basel). 2018;10(11):427.
Jiang Y, Zou S, Cao X. A simple dendrimer-aptamer based microfluidic platform for E. coli O157:H7 detection and signal intensification by rolling circle amplification[J]. Sensors Actuators B Chem. 2017;251:976–84.
He L, Shen Z, Cao Y, et al. A microfluidic chip based ratiometric aptasensor for antibiotic detection in foods using stir bar assisted sorptive extraction and rolling circle amplification[J]. Analyst. 2019;144(8):2755–64.
Wang X, Huang Y, Wu S, Duan N, Xu B, Wang Z. Simultaneous detection of Staphylococcus aureus and Salmonella typhimurium using multicolor time-resolved fluorescence nanoparticles as labels. Int J Food Microbiol. 2016;237:172–9.
Huang Y, Zhang H, Chen X, Wang X, Duan N, Wu S, et al. A multicolor time-resolved fluorescence aptasensor for the simultaneous detection of multiplex Staphylococcus aureus enterotoxins in the milk. Biosens Bioelectron. 2015;74:170–6.
Tu D, Liu L, Ju Q, Liu Y, Zhu H, Li R, et al. Time-resolved FRET biosensor based on amine-functionalized lanthanide-doped NaYF4 nanocrystals. Angew Chem Int Ed. 2011;50(28):6306–10.
Wang L, Bao J, Wang L, Zhang F, Li Y. One-pot synthesis and bioapplication of amine-functionalized magnetite nanoparticles and hollow nanospheres. Chem Eur J. 2006;12(24):6341–7.
Acknowledgments
This work was partly supported by the National Natural Science Foundation of China (31801647), “Chunhui Program” Cooperation Scientific Research of Ministry of Education of China (Z2016135), the Sichuan Science and Technology Program (2018JY0194, 2018SZ0340), the “Xihua Cup” Students Innovation and Entrepreneurship Project of Xihua University (2019082), and the Young Scholars Reserve Talent Program of Xihua University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 633 kb).
Rights and permissions
About this article
Cite this article
Huang, Y., Wang, C., Huo, Q. et al. A time-resolved luminescence aptasensor of ofloxacin based on rolling circle amplification and magnetic separation. Anal Bioanal Chem 412, 4555–4563 (2020). https://doi.org/10.1007/s00216-020-02708-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02708-3