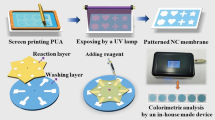
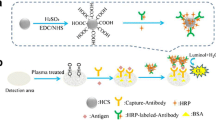
Abstract
Paper-based immunoassays are effective methods that employ microfluidic paper-based analytical devices (μPADs) for the rapid, simple, and accurate quantification of analytes in point-of-care diagnosis. In this study, we developed a wax-printed multilayered μPAD for the colorimetric detection of carcinoembryonic antigen (CEA), where the device contained a movable and rotatable detection layer to allow the μPAD to switch the state of the sample solutions, i.e., flowing or storing in the sensing zones. A smartphone with a custom-developed program served as an automated colorimetric reader to capture and analyze images from the μPAD, before calculating and displaying the test results. After optimizing the crucial conditions for the assay, the proposed method exhibited a wide linear dynamic range from 0.5 to 70 ng/mL, with a low CEA detection limit of 0.015 ng/mL. The clinical performance of this method was successfully validated using 50 positive and 40 negative human serum samples, thereby demonstrating the high sensitivity of 98.0% and specificity of 97.5% in the detection of CEA. The proposed method is greatly simplified compared with the cumbersome steps required for traditional immunoassays, but without any loss of accuracy and stability, as well as reducing the time needed to detect CEA. Complex and bulky instruments are replaced with a smartphone. The proposed detection platform could potentially be applied in point-of-care testing.

Graphical abstract






Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991;145(5):907–23.
Nakatsura T, Yoshitake Y, Senju S, Monji M, Komori H, Motomura Y, et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun. 2003;306(1):16–25.
Hayes DF, Bast RC, Desch CE, Fritsche H Jr, Kemeny NE, Jessup JM, et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst. 1996;88(20):1456–66.
Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. BMC Med. 2012;10(1):51.
Takahashi Y, Takeuchi T, Sakamoto J, Touge T, Mai M, Ohkura H, et al. The usefulness of CEA and/or CA19-9 in monitoring for recurrence in gastric cancer patients: a prospective clinical study. Gastric Cancer. 2003;6(3):142–5.
Lai I-R, Lee W-J, Huang M-T, Lin H-H. Comparison of serum CA72-4, CEA, TPA, CA19-9 and CA125 levels in gastric cancer patients and correlation with recurrence. Hepatogastroenterology. 2002;49(46):1157–60.
Ychou M, Duffour J, Kramar A, Gourgou S, Grenier J. Clinical significance and prognostic value of CA72-4 compared with CEA and CA19-9 in patients with gastric cancer. Dis Markers. 2000;16(3, 4):105–10.
Sun Z, Zhang N. Clinical evaluation of CEA, CA19-9, CA72-4 and CA125 in gastric cancer patients with neoadjuvant chemotherapy. World J Surg Oncol. 2014;12(1):397.
Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989;57(2):327–34.
Lequin RM. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin Chem. 2005;51(12):2415–8.
Zhou F, Wang M, Yuan L, Cheng Z, Wu Z, Chen H. Sensitive sandwich ELISA based on a gold nanoparticle layer for cancer detection. Analyst. 2012;137(8):1779–84.
Cai X, Weng S, Guo R, Lin L, Chen W, Zheng Z, et al. Ratiometric electrochemical immunoassay based on internal reference value for reproducible and sensitive detection of tumor marker. Biosens Bioelectron. 2016;81:173–80.
Wu J, Fu Z, Yan F, Ju H. Biomedical and clinical applications of immunoassays and immunosensors for tumor markers. TrAC Trends Anal Chem. 2007;26(7):679–88.
Jie G-F, Liu P, Zhang S-S. Highly enhanced electrochemiluminescence of novel gold/silica/CdSe-CdS nanostructures for ultrasensitive immunoassay of protein tumor marker. Chem Commun. 2010;46(8):1323–5.
Guo Z, Hao T, Du S, Chen B, Wang Z, Li X, et al. Multiplex electrochemiluminescence immunoassay of two tumor markers using multicolor quantum dots as labels and graphene asconductingbridge. Biosens Bioelectron. 2013;44:101–7.
Chakkarapani S, Zhang P, Ahn S, Kang S. Total internal reflection plasmonic scattering-based fluorescence-free nanoimmunosensor probe for ultra-sensitive detection of cancer antigen 125. Biosens Bioelectron. 2016;81:23–31.
Cheng AK, Su H, Wang YA, Yu H-Z. Aptamer-based detection of epithelial tumor marker mucin 1 with quantum dot-based fluorescence readout. Anal Chem. 2009;81(15):6130–9.
Yang J, Wang K, Xu H, Yan W, Jin Q, Cui D. Detection platforms for point-of-care testing based on colorimetric, luminescent and magnetic assays: a review. Talanta. 2019.
Hong L, Wang K, Yan W, Xu H, Chen Q, Zhang Y, et al. High performance immunochromatographic assay for simultaneous quantitative detection of multiplex cardiac markers based on magnetic nanobeads. Theranostics. 2018;8(22):6121.
Yan W, Wang K, Xu H, Huo X, Jin Q, Cui D. Machine learning approach to enhance the performance of MNP-labeled lateral flow immunoassay. Nano-Micro Letters. 2019;11(1):7.
Gao W, Huang H, Zhang Y, Zhu P, Yan X, Fan J, et al. Recombinase polymerase amplification-based assay for rapid detection of listeria monocytogenes in food samples. Food Anal Methods. 2017;10(6):1972–81.
Wang Y, Deng R, Zhang G, Li Q, Yang J, Sun Y, et al. Rapid and sensitive detection of the food allergen glycinin in powdered milk using a lateral flow colloidal gold immunoassay strip test. J Agric Food Chem. 2015;63(8):2172–8.
Lin LK, Uzunoglu A, Stanciu LA. Aminolated and thiolated PEG-covered gold nanoparticles with high stability and antiaggregation for lateral flow detection of bisphenol A. Small. 2018;14(10):1702828.
Quesada-González D, Jairo GA, Blake RC, Blake DA, Merkoçi A. Uranium (VI) detection in groundwater using a gold nanoparticle/paper-based lateral flow device. Sci Rep. 2018;8(1):16157.
Qin W, Wang K, Xiao K, Hou Y, Lu W, Xu H, et al. Carcinoembryonic antigen detection with “handing”-controlled fluorescence spectroscopy using a color matrix for point-of-care applications. Biosens Bioelectron. 2017;90:508–15.
Xiao K, Wang K, Qin W, Hou Y, Lu W, Xu H, et al. Use of quantum dot beads-labeled monoclonal antibody to improve the sensitivity of a quantitative and simultaneous immunochromatographic assay for neuron specific enolase and carcinoembryonic antigen. Talanta. 2017;164:463–9.
Serebrennikova K, Samsonova J, Osipov A. Hierarchical nanogold labels to improve the sensitivity of lateral flow immunoassay. Nano-micro letters. 2018;10(2):24.
Xie Y, Chen D, Lin S. Microfluidic electrochemical detection techniques of cancer biomarkers. Nano Biomed Eng. 2017;9(1):57–71.
He Q, Ma C, Hu X, Chen H. Method for fabrication of paper-based microfluidic devices by alkylsilane self-assembling and UV/O3-patterning. Anal Chem. 2013;85(3):1327–31.
Haller PD, Flowers CA, Gupta M. Three-dimensional patterning of porous materials using vapor phase polymerization. Soft Matter. 2011;7(6):2428–32.
Li F, Wang X, Liu J, Hu Y, He J. Double-layered microfluidic paper-based device with multiple colorimetric indicators for multiplexed detection of biomolecules. Sens Actuators B: Chem. 2019;288:266–73.
Wang X, Li F, Cai Z, Liu K, Li J, Zhang B, et al. Sensitive colorimetric assay for uric acid and glucose detection based on multilayer-modified paper with smartphone as signal readout. Anal Bioanal Chem. 2018;410(10):2647–55.
Abe K, Kotera K, Suzuki K, Citterio D. Inkjet-printed paperfluidic immuno-chemical sensing device. Anal Bioanal Chem. 2010;398(2):885–93.
Abe K, Suzuki K, Citterio D. Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal Chem. 2008;80(18):6928–34.
He Y, Wu Y, Xiao X, Fu J, Xue G. A low-cost and rapid microfluidic paper-based analytical device fabrication method: flash foam stamp lithography. RSC Adv. 2014;4(109):63860–5.
Xie L, Zi X, Zeng H, Sun J, Xu L, Chen S. Low-cost fabrication of a paper-based microfluidic using a folded pattern paper. Anal Chim Acta. 2019;1053:131–8.
Chen C, Wang P, Yen Y, Lin H, Fan Y, Wu S, et al. Fast analysis of ketamine using a colorimetric immunosorbent assay on a paper-based analytical device. Sens Actuators B: Chem. 2019;282:251–8.
Mazzu-Nascimento T, Morbioli GG, Milan LA, Donofrio FC, Mestriner CA, Carrilho E. Development and statistical assessment of a paper-based immunoassay for detection of tumor markers. Anal Chim Acta. 2017;950:156–61.
Gerold CT, Bakker E, Henry CS. Selective distance-based K+ quantification on paper-based microfluidics. Anal Chem. 2018;90(7):4894–900.
Zhu X, Xiong S, Zhang J, Zhang X, Tong X, Kong S. Improving paper-based ELISA performance through covalent immobilization of antibodies. Sens Actuators B: Chem. 2018;255:598–604.
Ma L, Nilghaz A, Choi JR, Liu X, Lu X. Rapid detection of clenbuterol in milk using microfluidic paper-based ELISA. Food Chem. 2018;246:437–41.
Pang B, Zhao C, Li L, Song X, Xu K, Wang J, et al. Development of a low-cost paper-based ELISA method for rapid Escherichia coli O157: H7 detection. Anal Biochem. 2018;542:58–62.
Hou Y, Wang K, Xiao K, Qin W, Lu W, Tao W, et al. Smartphone-based dual-modality imaging system for quantitative detection of color or fluorescent lateral flow immunochromatographic strips. Nanoscale Res Lett. 2017;12(1):291.
Srinivasan B, O’Dell D, Finkelstein JL, Lee S, Erickson D, Mehta S. IronPhone: mobile device-coupled point-of-care diagnostics for assessment of iron status by quantification of serum ferritin. Biosens Bioelectron. 2018;99:115–21.
Hou Y, Wang K, Yang M, Qin W, Xiao K, Yan W. Smartphone-based fluorescent diagnostic system for immunochromatographic chip. Nano Biomed Eng. 2017;9(1).
Sekine Y, Kim SB, Zhang Y, Bandodkar AJ, Xu S, Choi J, et al. A fluorometric skin-interfaced microfluidic device and smartphone imaging module for in situ quantitative analysis of sweat chemistry. Lab Chip. 2018;18(15):2178–86.
Lopez-Ruiz N, Curto VF, Erenas MM, Benito-Lopez F, Diamond D, Palma AJ, et al. Smartphone-based simultaneous pH and nitrite colorimetric determination for paper microfluidic devices. Anal Chem. 2014;86(19):9554–62.
Dai X, Rasamani KD, Hu F, Sun Y. Mesoporous SiO2 nanoparticles: a unique platform enabling sensitive detection of rare earth ions with smartphone camera. Nano-micro letters. 2018;10(4):55.
Kjellgren H, Gällstedt M, Engström G, Järnström L. Barrier and surface properties of chitosan-coated greaseproof paper. Carbohydr Polym. 2006;65(4):453–60.
Verma MS, Tsaloglou M-N, Sisley T, Christodouleas D, Chen A, Milette J, et al. Sliding-strip microfluidic device enables ELISA on paper. Biosens Bioelectron. 2018;99:77–84.
Funding
We are grateful for the financial support by the National Key Research and Development Program of China (Grant Nos. 2017FYA0205303 and 2017FYA0205301), the National Natural Science Foundation of China (Grant Nos. 81571835 and 81672247), and the Shanghai Science and Technology Fund (No. 15DZ225200), and the funding of SJTU (Nos. ZH2018QNA03 and YG2019QNB09).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 615 kb)
Rights and permissions
About this article
Cite this article
Wang, K., Yang, J., Xu, H. et al. Smartphone-imaged multilayered paper-based analytical device for colorimetric analysis of carcinoembryonic antigen. Anal Bioanal Chem 412, 2517–2528 (2020). https://doi.org/10.1007/s00216-020-02475-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02475-1