Abstract
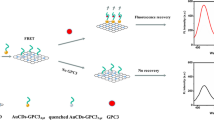
A versatile nanocomposite was simply prepared based upon the electrostatic adsorption of positively charged gold nanoparticles with negatively charged graphene oxide (nano-gold@GO), and utilized as a novel fluorescence quenching platform for ultrasensitive detection of adenosine triphosphate (ATP). In the designed system, DNA-stabilized Ag nanoclusters (DNA/AgNCs) were used as fluorescent probes, DNA duplex was formed in the presence of ATP, and they can electrostatically adsorb onto the surface of nano-gold@GO to quench the fluorescence signal. Upon the addition of exonuclease III (Exo III), the DNA duplex would be hydrolyzed into DNA fragments and resulted in the recovery of the fluorescence signals due to the diffusion of AgNCs away from nano-gold@GO. Based on these, sensitive detection of ATP was realized with a detection range of 5.0 pM–20 nM. Notably, a good recovery in the range of 94–104% was obtained when detecting ATP in human serum samples, indicating a promising application value in early disease diagnosis.

A functional positively charged nano-gold@graphene oxide was fabricated and utilized as an enhanced fluorescence quenching platform for the detection of ATP, coupled with exonuclease III-assisted signal amplification.






Similar content being viewed by others
References
Zhou Y, Tozzi F, Chen J, Fan F, Xia L, Wang J, et al. Intracellular ATP levels are a pivotal determinant of chemoresistance in colon cancer cells. Cancer Res. 2012;72:304–14.
Gourine AV, Enrique L, Nicholas D, Michael SK. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–11.
Lu C, Wang F, Willner I. Amplified optical aptasensors through the endonuclease-stimulated regeneration of the analyte. Chem Sci. 2012;3:2616–22.
Hu P, Zhu C, Jin L, Dong S. An ultrasensitive fluorescent aptasensor for adenosine detection based on exonuclease III assisted signal amplification. Biosens Bioelectron. 2012;34:83–7.
Fan D, Zhu X, Zhai Q, Wang E, Dong S. Polydopamine nanotubes as an effective fluorescent quencher for highly sensitive and selective detection of biomolecules assisted with exonuclease III amplification. Anal Chem. 2016;88:9158–65.
Wu F, Liu W, Yang S, Yao Q, Chen Y, Weng X, et al. An aptamer-based ligation-triggered rolling circle amplification strategy for ATP detection and imaging in situ. J Photoch Photobio A. 2018;355:114–9.
Ding X, Cheng W, Li Y, Wu J, Li X, Cheng Q, et al. An enzyme-free surface plasmon resonance biosensing strategy for detection of DNA and small molecule based on nonlinear hybridization chain reaction. Biosens Bioelectron. 2017;87:345–51.
Li X, Wang Y, Wang L, Wei Q. A surface plasmon resonance assay coupled with a hybridization chain reaction for amplified detection of DNA and small molecules. Chem Commun. 2014;50:5049–52.
Li X, Peng Y, Chai Y, Yuan R, Xiang Y. A target responsive aptamer machine for label-free and sensitive non-enzymatic recycling amplification detection of ATP. Chem Commun. 2016;52:3673–6.
Luo J, Shen X, Li B, Li X, Zhou X. Signal amplification by strand displacement in a carbon dot based fluorometric assay for ATP. Microchim Acta. 2018;185:392.
Dai J, He H, Duan Z, Guo Y, Xiao D. Self-replicating catalyzed hairpin assembly for rapid signal amplification. Anal Chem. 2017;89:11971–5.
Kong R, Song Z, Meng H, Zhang X, Shen G, Yu R. A label-free electrochemical biosensor for highly sensitive and selective detection of DNA via a dual-amplified strategy. Biosens Bioelectron. 2014;54:442–7.
Xu J, Yu H, Hu Y, Chen M, Shao S. A gold nanoparticle-based fluorescence sensor for high sensitive and selective detection of thiols in living cells. Biosens Bioelectron. 2016;75:1–7.
Samanta A, Zhou Y, Zou S, Yan H, Liu Y. Fluorescence quenching of quantum dots by gold nanoparticles: a potential long range spectroscopic ruler. Nano Lett. 2014;14:5052–7.
Xing T, Zhao J, Weng G, Zhu J, Li J, Zhao J. Specific detection of carcinoembryonic antigen based on fluorescence quenching of hollow porous gold nanoshells with roughened surface. ACS Appl Mater Interfaces. 2017;9:36632–41.
Miao X, Wang W, Kang T, Liu J, Shiu K, Leung C, et al. Ultrasensitive electrochemical detection of miRNA-21 by using an iridium (III) complex as catalyst. Biosens Bioelectron. 2016;86:454–8.
Li Z, Miao X, Xing K, Peng X, Zhu A, Ling L. Ultrasensitive electrochemical sensor for Hg2+ by using hybridization chain reaction coupled with Ag@Au core–shell nanoparticles. Biosens Bioelectron. 2016;80:339–43.
Anger P, Bharadwaj P, Novotny L. Enhancement and quenching of single-molecule fluorescence. Phys Rev Lett. 2006;96:113002.
Cui X, Zhu L, Wu J, Hou Y, Wang P, Wang Z, et al. A fluorescent biosensor based on carbon dots-labeled oligodeoxyribonucleotide and graphene oxide for mercury (II) detection. Biosens Bioelectron. 2015;63:506–12.
Li F, Chao J, Li Z, Xing S, Su S, Li X, et al. Graphene oxide-assisted nucleic acids assays using conjugated polyelectrolytes-based fluorescent signal transduction. Anal Chem. 2015;87:3877–83.
Zhou Q, Lin Y, Zhang K, Li M, Tang D. Reduced graphene oxide/BiFeO3 nanohybrids-based signal-on photoelectrochemical sensing system for prostate-specific antigen detection coupling with magnetic microfluidic device. Biosens Bioelectron. 2018;101:146–52.
Zhang K, Lv S, Lin Z, Tang D. CdS:Mn quantum dot-functionalized g-C3N4 nanohybrids as signal-generation tags for photoelectrochemical immunoassay of prostate specific antigen coupling DNAzyme concatamer with enzymatic biocatalytic precipitation. Biosens Bioelectron. 2017;95:34–40.
Chen X, Yu S, Yang L, Wang J, Jiang C. Fluorescence and visual detection of fluoride ions using a photoluminescent graphene oxide paper sensor. Nanoscale. 2016;8:13669–77.
Li Z, Xue N, Ma H, Cheng Z, Miao X. An ultrasensitive and switch-on platform for aflatoxin B1 detection in peanut based on the fluorescence quenching of graphene oxide-gold nanocomposites. Talanta. 2018;181:346–51.
Wang Q, Li Q, Yang X, Wang K, Du S, Zhang H, et al. Graphene oxide–gold nanoparticles hybrids-based surface plasmon resonance for sensitive detection of microRNA. Biosens Bioelectron. 2016;77:1001–7.
Myung S, Park J, Lee H, Kim KS, Hong S. Ambipolar memory devices based on reduced graphene oxide and nanoparticles. Adv Mater. 2010;22:2045–9.
Huang K, Niu D, Liu X, Wu Z, Fan Y, Chang Y, et al. Direct electrochemistry of catalase at amine-functionalized graphene/gold nanoparticles composite film for hydrogen peroxide sensor. Electrochim Acta. 2011;56:2947–53.
Cui P, Seo S, Lee J, Wang L, Lee E, Min M, et al. Nonvolatile memory device using gold nanoparticles covalently bound to reduced graphene oxide. ACS Nano. 2011;5:6826–33.
Liu G, Qi M, Zhang Y, Cao C, Goldys EM. Nanocomposites of gold nanoparticles and graphene oxide towards an stable label-free electrochemical immunosensor for detection of cardiac marker troponin-I. Anal Chim Acta. 2016;909:1–8.
Yun Y, Song K. Preparation and characterization of graphene oxide encapsulated gold nanoparticles. J Nanosci Nanotechnol. 2013;13:7376–80.
Khalil I, Julkapli NM, Yehye WA, Basirun WJ, Bhargava SK. Graphene–gold nanoparticles hybrid-synthesis, functionalization, and application in a electrochemical and surface-enhanced raman scattering biosensor. Materials. 2016;9:406.
Mao S, Lu G, Yu K, Bo Z, Chen J. Specific protein detection using thermally reduced graphene oxide sheet decorated with gold nanoparticle-antibody conjugates. Adv Mater. 2010;22:3521–6.
Miao X, Cheng Z, Ma H, Li Z, Xue N, Wang P. Label-free platform for microRNA detection based on the fluorescence quenching of positively charged gold nanoparticles to silver nanoclusters. Anal Chem. 2017;90:1098–103.
Miao X, Li Z, Zhu A, Feng Z, Tian J, Peng X. Ultrasensitive electrochemical detection of protein tyrosine kinase-7 by gold nanoparticles and methylene blue assisted signal amplification. Biosens Bioelectron. 2016;83:39–44.
Qu L, Wang N, Xu H, Wang W, Liu Y, Kuo L, et al. Gold nanoparticles and g-C3N4-intercalated graphene oxide membrane for recyclable surface enhanced raman scattering. Adv Funct Mater. 2017;27:1701714.
Moon H, Kumar D, Kim H, Sim C, Chang J, Kim J, et al. Amplified photoacoustic performance and enhanced photothermal stability of reduced graphene oxide coated gold nanorods for sensitive photoacoustic imaging. ACS Nano. 2015;9:2711–9.
Wang J, Wang X, Wu S, Song J, Zhao Y, Ge Y, et al. Fabrication of highly catalytic silver nanoclusters/graphene oxide nanocomposite as nanotag for sensitive electrochemical immunoassay. Anal Chim Acta. 2016;906:80–8.
Pu W, Zhang L, Huang C. Graphene oxide as a nano-platform for ATP detection based on aptamer chemistry. Anal Methods. 2012;4:1662–6.
Ning Y, Wei K, Cheng L, Hu J, Xiang Q. Fluorometric aptamer based determination of adenosine triphosphate based on deoxyribonuclease i-aided target recycling and signal amplification using graphene oxide as a quencher. Microchim Acta. 2017;184:1847–54.
Wang Y, Liu J, Duan L, Liu S, Jiang J. Aptamer-based fluorometric determination of ATP by using target-cycling strand displacement amplification and copper nanoclusters. Microchim Acta. 2017;184:4183–8.
Cheng X, Cen Y, Xu G, Wei F, Shi M, Xu X, et al. Aptamer based fluorometric determination of ATP by exploiting the FRET between carbon dots and graphene oxide. Microchim Acta. 2018;185:144.
Zhang R, Sun J, Ji J, Pi F, Xiao Y, Zhang Y, et al. A novel “OFF-ON” biosensor based on nanosurface energy transfer between gold nanocrosses and graphene quantum dots for intracellular ATP sensing and tracking. Sensors Actuators B Chem. 2019;282:910–6.
El Kurdi R, Patra D. Nanosensing of ATP by fluorescence recovery after surface energy transfer between rhodamine B and curcubit [7] uril-capped gold nanoparticles. Microchim Acta. 2018;185:349.
Song Q, Peng M, Wang L, He D, Ouyang J. A fluorescent aptasensor for amplified label-free detection of adenosine triphosphate based on core–shell Ag@SiO2 nanoparticles. Biosens Bioelectron. 2016;77:237–41.
Ma K, Wang H, Li H, Wang S, Li X, Xu B, et al. A label-free aptasensor for turn-on fluorescent detection of ATP based on AIE-active probe and water-soluble carbon nanotubes. Sensors Actuators B Chem. 2016;230:556–8.
Song Q, Wang R, Sun F, Chen H, Wang Z, Na N, et al. A nuclease-assisted label-free aptasensor for fluorescence turn-on detection of ATP based on the in situ formation of copper nanoparticles. Biosens Bioelectron. 2017;87:760–3.
Acknowledgments
The authors wish to acknowledge the Natural Science Foundation of Xuzhou City (KC18140) and the project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Written informed consent was obtained from the patient for research use. The study was approved by the Jiangsu Normal University ethics committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xue, N., Wu, S., Li, Z. et al. One-step and ultrasensitive ATP detection by using positively charged nano-gold@graphene oxide as a versatile nanocomposite. Anal Bioanal Chem 412, 2487–2494 (2020). https://doi.org/10.1007/s00216-020-02470-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02470-6