Abstract
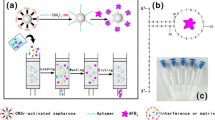
We have developed an aptamer affinity column (AAC) for the purification and enrichment of trace aflatoxin B1 (AFB1) and aflatoxin B2 (AFB2) in genuine agro-products through the covalent conjugation of amino modified aptamer and NHS-activated Sepharose. The coupling and working conditions found to be suitable for this AFB-AAC were examined in regard to coupling time (2 min), loading volume (30 mL), and the methanol concentration (< 10%) used in the loading step. The performance of AFB-AAC was then further evaluated in terms of capacity (329.1 ± 13.7 ng for AFB1 and 162.5 ± 8.9 ng for AFB2), selectivity (excellent), reusability (twenty-three times for AFB1 and twelve times for AFB2), and repeatability (92.7% ± 2.9% for AFB1 and 71.5% ± 3.4% for AFB2). Furthermore, the AAC clean-up combined with HPLC-FLD demonstrated excellent linearity over a wide range, good sensitivity with an LOD of 50 pg mL−1 for AFB1 and 15 pg mL−1 for AFB2, and acceptable recovery with different spiking levels in different matrices. Finally, the AAC was successfully applied to analyte AFB1 and AFB2 in four types of agro-products as well as a maize flour reference material, and the results were found to be in accordance with those of commercial IACs. This study provides a reference for the analysis of other trace analytes by merely changing the corresponding aptamer and represents a strong contender for immune affinity columns.

An aptamer affinity column for purification and enrichment of aflatoxin B1 and aflatoxin B2 in agro-products with the aid of HPLC-FLD and a post-column photochemical derivatization reactor





Similar content being viewed by others
References
Tamura M, Takahashi A, Uyama A, Mochizuki N. A method for multiple mycotoxin analysis in wines by solid phase extraction and multifunctional cartridge purification, and ultra-high-performance liquid chromatography coupled to tandem mass spectrometry. Toxins. 2012;4:476–86.
Shim WB, Kim MJ, Mun H, Kim MG. An aptamer-based dipstick assay for the rapid and simple detection of aflatoxin B1. Biosens Bioelectron. 2014;62:288–94.
Li QF, Lv SZ, Lu MH, Lin ZZ, Tang DP. Potentiometric competitive immunoassay for determination of aflatoxin B1 in food by using antibody-labeled gold nanoparticles. Microchim Acta 2016; 183(10): 2815–2822.
Atar N, Eren T, Yola ML. A molecular imprinted SPR biosensor for sensitive determination of citrinin in red yeast rice. Food Chem. 2015;184:7–11.
Guo PQ, Yang W, Hu H, Wang YT, Li P. Rapid detection of aflatoxin B1 by dummy template molecularly imprinted polymer capped CdTe quantum dots. Anal Bioanal Chem. 2019;411:2607–17.
Al-Jawdah A, Nabok A, Abu-Ali H, Catanante G, Marty JL, Szekacs A. Highly sensitive label-free in vitro detection of aflatoxin B1 in an aptamer assay using optical planar waveguide operating as a polarization interferometer. Anal Bioanal Chem. 2019;411(29):7717–7724.
Hajian R, Ensafi AA. Determination of aflatoxins B1 and B2 by adsorptive cathodic stripping voltammetry in groundnut. Food Chem. 2009;115:1034–7.
IARC. A review of human carcinogens: chemical agents and related occupations. IARC Monogr Eval Carcinog Risks Hum, 2012; 100F.
Zhang L, Dou XW, Kong WJ, Liu CM, Han X, Yang MH. Assessment of critical points and development of a practical strategy to extend the applicable scope of immunoaffinity column cleanup for aflatoxin detection in medicinal herbs. J Chromatogr A. 2017;1483:56–63.
Var I, Kabak B, Gök F. Survey of Aflatoxin B1 in helva, a traditional Turkish food, by TLC. Food Control. 2007;18:59–62.
Sun LL, Wu LQ, Zhao Q. Aptamer based surface plasmon resonance sensor for aflatoxin B1. Microchim Acta. 2017;184(8):2605–10.
Wu L, Ding F, Yin WM, Ma J, Wang BR, Nie AX, et al. From electrochemistry to electroluminescence: development and application in a ratiometric aptasensor for aflatoxin B1. Anal Chem. 2017;89:7578–85.
Wang C, Zhao Q. A competitive thrombin-linked aptamer assay for small molecule: aflatoxin B1. Anal Bioanal Chem. 2019;411:6637–44.
Wu XM, Hu J, Zhu BH, Lu L, Huang XD, Pang DW. Aptamer-targeted magnetic nanospheres as a solid-phase extraction sorbent for determination of ochratoxin A in food samples. J Chromatogr A. 2011;1218:7341–6.
Liu HM, Lu AX, Fu HL, Li BR, Yang MH, Wang JH, et al. Affinity capture of aflatoxin B1 and B2 by aptamer-functionalized magnetic agarose microspheres prior to their determination by HPLC. Microchim Acta. 2018;185:326.
AOAC–IUPAC Method. 49.2.18A AOAC official method 2005.08 aflatoxins in corn, raw peanuts, and peanut butter. J AOAC Int, 2006; 89: 678–679.
European Pharmacopoeia. The European directorate for the quality of medicines 8th edition 8.0. Council of Europe, Strasbourg, pp. 2014; 276–278.
Chinese Pharmacopoeia Commission. Chinese pharmacopoeia, vol IV, 2015th edn. Chinese Medicine Science and Technology Press, Beijing, 2015; 224–225.
United States Pharmacopeial Convention. Chapter 561: articles of botanical origin, second supplement to USP 38-NF 33. Rockville. 2014:7–9.
Mairal T, Ozalp VC, Lozano Sanchez P, Mir M, Katakis I, O’Sullivan CK. Aptamers: molecular tools for analytical applications. Anal Bioanal Chem 2008; 390: 989–1007.
Rhouati A, Paniel N, Meraihi Z, Marty JL. Development of an oligosorbent for detection of ochratoxin A. Food Control. 2011;22:1790–5.
Jayasena SD. Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem. 1999;45:1628–50.
Luzi E, Minunni M, Tombelli S, Mascini M. New trends in affinity sensing: aptamers for ligand binding. TrAC Trends Anal Chem. 2003;22:810–8.
Peyrin E. Nucleic acid aptamer molecular recognition principles and application in liquid chromatography and capillary electrophoresis. J Sep Sci. 2009;32:1531–6.
Schax E, Lönne M, Scheper T, Belkin S, Walter JG. Aptamer-based depletion of small molecular contaminants: a case study using ochratoxin A. Biotechnol Bioproc E. 2015;20:1016–25.
Liu HM, Luan YX, Lu AX, Li BR, Yang MH, Wang JH. An oligosorbent-based aptamer affinity column for selective extraction of aflatoxin B2 prior to HPLC with fluorometric detection. Microchim Acta. 2018;185:71.
Cruz-Aguado JA, Penner G. Determination of ochratoxin A with a DNA Aptamer. J Agr Food Chem. 2008;56:10456–61.
De-Girolamo A, McKeague M, Miller JD, DeRosa MC, Visconti A. Determination of ochratoxin A in wheat after clean-up through a DNA aptamer-based solid phase extraction column. Food Chem. 2011;127:1378–84.
Chapuis-Hugon F, du-Boisbaudry A, Madru B, Pichon V. New extraction sorbent based on aptamers for the determination of ochratoxin A in red wine. Anal Bioanal Chem. 2011, 400: 1199–1207.
Ali WH, Pichon V. Characterization of oligosorbents and application to the purification of ochratoxin A from wheat extracts. Anal Bioanal Chem. 2014;406:1233–40.
Yang XH, Kong WJ, Hu YC, Yang MH, Huang LQ, Zhao M, et al. Aptamer-affinity column clean-up coupled with ultra high performance liquid chromatography and fluorescence detection for the rapid determination of ochratoxin a in ginger powder. J Sep Sci. 2014;37:853–60.
Le Chryseis L, Cruz-Aguado JA, Penner GA. DNA ligands for aflatoxin and zearalenone. USA patent PTC/CA2010/001292. 2012.
Wang M, Jiang N, Xian H, Wei DZ, Shi L, Feng XY. A single-step solid phase extraction for the simultaneous determination of 8 mycotoxins in fruits by ultra-high performance liquid chromatography tandem mass spectrometry. J Chromatogr A. 2016;1429:22–9.
Jiang DM, Wei DZ, Wang LQ, Ma S, Du YF, Wang M. Multiwalled carbon nanotube for one-step cleanup of 21 mycotoxins in corn and wheat prior to ultraperformance liquid chromatography–tandem mass spectrometry analysis. Toxins. 2018;10:409.
Hamula CLA, Guthrie JW, Zhang HQ, Li XF, Le XC. Selection and analytical applications of aptamers. TrAC Trends Anal Chem. 2006;25:681–91.
European Commission. No 401/2006. Official journal. https://www.fsvps.ru/fsvps-docs/ru/usefulinf/fles/es401-2006.pdf/, 2006 Accessed date, 3 March 2018.
Codex Alimentarius Commission. Procedural Manual 24th Edition Codex Alimentarius Commission, Rome, 2015; 72–75.
European Commission. No 165-2010 amending Regulation (EC) No 1881-2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off J Eur Union. 2010;L50:8–12.
Acknowledgements
The authors are very grateful for powerful suggestions from anonymous referees. We also thank the support from the science and technology innovation special construction funded program of National Natural Science Foundation of China (81803712), Beijing Academy of Agriculture and Forestry Sciences (KJCX20170401), Beijing Agricultural Forestry Academy Youth Foundation (QNJJ201630), Beijing Municipal Natural Science Foundation (7192026), and Beijing Excellent Talent Training Fund (2017000020060G131).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, H., Zhao, Y., Lu, A. et al. An aptamer affinity column for purification and enrichment of aflatoxin B1 and aflatoxin B2 in agro-products. Anal Bioanal Chem 412, 895–904 (2020). https://doi.org/10.1007/s00216-019-02300-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02300-4