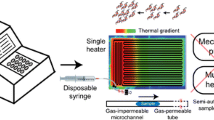
Abstract
The design and fabrication of a continuous-flow μPCR device with very short amplification time and low power consumption are presented. Commercially available, 4-layer printed circuit board (PCB) substrates are employed, with in-house designed yet industrially manufactured embedded Cu micro-resistive heaters lying at very close distance from the microfluidic network, where DNA amplification takes place. The 1.9-m-long microchannel in combination with desirably high flow velocities (for fast amplification) challenged the robustness of the sealing that was overcome with the development of a novel bonding method rendering the microdevice robust even at extreme pressure drops (12 bars). The proposed fabrication methods are PCB compatible, allowing for mass and reliable production of the μPCR device in the established PCB industry. The μPCR chip was successfully validated during the amplification of two different DNA fragments (and with different target DNA copies) corresponding to the exon 20 of the BRCA1 gene, and to the plasmid pBR322, a commonly used cloning vector in E. coli. Successful DNA amplification was demonstrated at total reaction times down to 2 min, with a power consumption of 2.7 W, rendering the presented μPCR one of the fastest and lowest power-consuming devices, suitable for implementation in low-resource settings. Detailed numerical calculations of the DNA residence time distributions, within an acceptable temperature range for denaturation, annealing, and extension, performed for the first time in the literature, provide useful information regarding the actual on-chip PCR protocol and justify the maximum volumetric flow rate for successful DNA amplification. The calculations indicate that the shortest amplification time is achieved when the device is operated at its enzyme kinetic limit (i.e., extension rate).

Graphical abstract





Similar content being viewed by others
References
Arora A, Simone G, Salieb-Beugelaar GB, Kim JT, Manz A. Latest developments in micro total analysis systems. Anal Chem. 2010;82(12):4830–47.
Trietsch SJ, Hankemeier T, van der Linden HJ. Lab-on-a-chip technologies for massive parallel data generation in the life sciences: a review. Chemometr Intell Lab. 2011;108(1):64–75.
Romao VC, Martins SAM, Germano J, Cardoso FA, Cardoso S, Freitas PP. Lab-on-chip devices: gaining ground losing size. ACS Nano. 2017;11(11):10659–64.
Ahmad F, Hashsham SA. Miniaturized nucleic acid amplification systems for rapid and point-of-care diagnostics: a review. Anal Chim Acta. 2012;733:1–15.
Chouler J, Di Lorenzo M. Water quality monitoring in developing countries; can microbial fuel cells be the answer? Biosensors. 2015;5(3):450–70.
Voetsch AC, Van Gilder TJ, Angulo FJ, Farley MM, Shallow S, Marcus R, et al. FoodNet estimate of the burden of illness caused by nontyphoidal Salmonella infections in the United States. Clin Infect Dis. 2004;38(Supplement_3):S127–S34.
Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50(6):882–9.
Zhao X, Lin C-W, Wang J, Oh DH. Advances in rapid detection methods for foodborne pathogens. J Microbiol Biotechnol. 2014;24(3):297–312.
Pandey CM, Augustine S, Kumar S, Kumar S, Nara S, Srivastava S, et al. Microfluidics based point-of-care diagnostics. Biotechnol Adv. 2018;13(1):1700047.
Bruijns B, van Asten A, Tiggelaar R, Gardeniers H. Microfluidic devices for forensic DNA analysis: a review. Biosensors. 2016;6(3):41.
Khalid N, Kobayashi I, Nakajima M. Recent lab-on-chip developments for novel drug discovery. Wiley Interdiscip Rev Syst Biol Med. 2017;9(4):e1381.
Mullis K, Faloona F, Scharf S, Saiki R, Horn G, Erlich H, editors. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction Cold Spring Harbor symposia on quantitative biology. Cold Spring: Harbor Laboratory Press; 1986.
Roper MG, Easley CJ, Landers JP. Advances in polymerase chain reaction on microfluidic chips. Anal Chem. 2005;77(12):3887–94.
Zhang C, Xu J, Ma W, Zheng W. PCR microfluidic devices for DNA amplification. Biotechnol Adv. 2006;24(3):243–84.
Zhang C, Xing D. Miniaturized PCR chips for nucleic acid amplification and analysis: latest advances and future trends. Nucleic Acids Res. 2007;35(13):4223–37.
Park S, Zhang Y, Lin S, Wang T-H, Yang S. Advances in microfluidic PCR for point-of-care infectious disease diagnostics. Biotechnol Adv. 2011;29(6):830–9.
Zhang Y, Ozdemir P. Microfluidic DNA amplification—a review. Anal Chim Acta. 2009;638(2):115–25.
Northrup MA, Gonzalez C, Hadley D, Hills RF, Landre P, Lehew S, et al., editors. A mems-based miniature DNA analysis system. Transducers '95 1995 25-29 June 1995.
Xiang Q, Xu B, Fu R, Li D. Real time PCR on disposable PDMS chip with a miniaturized thermal cycler. Biomed Microdevices. 2005;7(4):273–9.
Kopp MU, De Mello AJ, Manz A. Chemical amplification: continuous-flow PCR on a chip. Science. 1998;280(5366):1046–8.
Wang H, Chen J, Zhu L, Shadpour H, Hupert ML, Soper SA. Continuous flow thermal cycler microchip for DNA cycle sequencing. Anal Chem. 2006;78(17):6223–31.
Moschou D, Vourdas N, Kokkoris G, Papadakis G, Parthenios J, Chatzandroulis S, et al. All-plastic, low-power, disposable, continuous-flow PCR chip with integrated microheaters for rapid DNA amplification. Sensors Actuators B Chem. 2014;199:470–8.
Sun Y, Kwok Y-C, Foo-Peng Lee P, Nguyen N-T. Rapid amplification of genetically modified organisms using a circular ferrofluid-driven PCR microchip. Anal Bioanal Chem. 2009;394(5):1505–8.
Tsung-Min H, Ching-Hsing L, Gwo-Bin L, Chia-Sheng L, Fu-Chun H. A micromachined low-power-consumption portable PCR system. J Med Biol Eng. 2006;26(1):43–9.
Papadopoulos VE, Kokkoris G, Kefala IN, Tserepi A. Comparison of continuous-flow and static-chamber μPCR devices through a computational study: the potential of flexible polymeric substrates. Microfluid Nanofluid. 2015;19(4):867–82.
Volpatti LR, Yetisen AK. Commercialization of microfluidic devices. Trends Biotechnol. 2014;32(7):347–50.
Mohammed MI, Haswell S, Gibson I. Lab-on-a-chip or chip-in-a-lab: challenges of commercialization lost in translation. Proc Technol. 2015;20(Supplement C):54–9.
Duchesne L, Lacombe K. Innovative technologies for point-of-care testing of viral hepatitis in low-resource and decentralized settings. J Viral Hepat. 2018;25(2):108–17.
Walsh DI, Kong DS, Murthy SK, Carr PA. Enabling microfluidics: from clean rooms to makerspaces. Trends Biotechnol. 2017;35(5):383–92.
Merkel T, Graeber M, Pagel L. New technology for fluidic microsystems based on PCB technology. Sens Actuators A Phys. 1999;77(2):98–105.
Gaßmann S, Ibendorf I, Pagel L. Realization of a flow injection analysis in PCB technology. Sens Actuators A Phys. 2007;133(1):231–5.
Aracil C, Perdigones F, Moreno JM, Luque A, Quero JM. Portable lab-on-PCB platform for autonomous micromixing. Microelectron Eng. 2015;131:13–8.
Moschou D, Tserepi A. The lab-on-PCB approach: tackling the μTAS commercial upscaling bottleneck. Lab Chip. 2017;17(8):1388–405.
Nguyen N-T, Huang X. Miniature valveless pumps based on printed circuit board technique. Sens Actuators A Phys. 2001;88(2):104–11.
Ingle AP, Duran N, Rai M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: a review. Appl Microbiol Biotechnol. 2014;98(3):1001–9.
Li J, Wang Y, Dong E, Chen H. USB-driven microfluidic chips on printed circuit boards. Lab Chip. 2014;14(5):860–4.
Metz S, Holzer R, Renaud P. Polyimide-based microfluidic devices. Lab Chip. 2001;1(1):29–34.
Mavraki E, Moschou D, Kokkoris G, Vourdas N, Chatzandroulis S, Tserepi A. A continuous flow μPCR device with integrated microheaters on a flexible polyimide substrate. Procedia Eng. 2011;25:1245–8.
Wangler N, Gutzweiler L, Kalkandjiev K, Müller C, Mayenfels F, Reinecke H, et al. High-resolution permanent photoresist laminate TMMF for sealed microfluidic structures in biological applications. J Micromech Microeng. 2011;21(9):095009.
Wu LL, Marshall LA, Babikian S, Han CM, Santiago JG, Bachman M, editors. A printed circuit board based microfluidic system for point-of-care diagnostics applications. 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences (MicroTAS) 2011.
Wu LL, Babikian S, Li GP, Bachman M, editors. Microfluidic printed circuit boards. Proceedings - Electronic Components and Technology Conference 2011.
Vasilakis N, Moschou D, Carta D, Morgan H, Prodromakis T. Long-lasting FR-4 surface hydrophilisation towards commercial PCB passive microfluidics. Appl Surf Sci. 2016;368:69–75.
Papadopoulos VE, Kefala IN, Kaprou G, Kokkoris G, Moschou D, Papadakis G, et al. A passive micromixer for enzymatic digestion of DNA. Microelectron Eng. 2014;124:42–6.
Kefala IN, Papadopoulos VE, Karpou G, Kokkoris G, Papadakis G, Tserepi A. A labyrinth split and merge micromixer for bioanalytical applications. Microfluid Nanofluid. 2015;19(5):1047–59.
Kaprou G, Papadakis G, Papageorgiou D, Kokkoris G, Papadopoulos V, Kefala I, et al. Miniaturized devices for isothermal DNA amplification addressing DNA diagnostics. Microsyst Technol. 2016;22(7):1529–34.
Temiz Y, Lovchik RD, Kaigala GV, Delamarche E. Lab-on-a-chip devices: how to close and plug the lab? Microelectron Eng. 2015;132:156–75.
Becker H, Gärtner C. Polymer microfabrication technologies for microfluidic systems. Anal Bioanal Chem. 2008;390(1):89–111.
Kaprou G, Papadakis G, Kokkoris G, Papadopoulos V, Kefala I, Papageorgiou D, et al., editors. Miniaturized devices towards an integrated lab-on-a-chip platform for DNA diagnostics. Progress in Biomedical Optics and Imaging - Proceedings of SPIE; 2015.
Cao Q, Kim M-C, Klapperich C. Plastic microfluidic chip for continuous-flow polymerase chain reaction: simulations and experiments. Biotechnol Adv. 2011;6(2):177–84.
Ltd E. Technical terms and abbreviations. Available from: https://www.eurocircuits.com/technical-terms-and-abbreviations/.
Tserepi A., Chatzandroulis S., Kaprou G., Kokkoris G., Ellinas K., Papageorgiou D., inventorMicrofluidic reactors and process for their production. Greece patent GRA 20170100305 2017 30.06.2017.
Tserepi A., Chatzandroulis S., Kaprou G., Kokkoris G., Ellinas K., Papageorgiou D., inventorMicrofluidic reactors and process for their production patent 18386020.4-1101. 2018 29.06.18.
Vorkas PA, Christopoulos K, Kroupis C, Lianidou ES. Mutation scanning of exon 20 of the BRCA1 gene by high-resolution melting curve analysis. Clin Biochem. 2010;43(1–2):178–85.
KAPABIOSYSTEMS.https://www.kapabiosystems.com/product-applications/products/pcr-2/kapa2g-fast-pcr-kits/.
Leonard WF. Yu HY. Thermoelectric power of thin copper films. J Appl Phys. 1973;44(12):5320–3.
Kim YS. Microheater-integrated single gas sensor array chip fabricated on flexible polyimide substrate. Sensors Actuators B Chem. 2006;114(1):410–7.
Shen K, Chen X, Guo M, Cheng J. A microchip-based PCR device using flexible printed circuit technology. Sensors Actuators B Chem. 2005;105(2):251–8.
Wheeler EK, Benett W, Stratton P, Richards J, Chen A, Christian A, et al. Convectively driven polymerase chain reaction thermal cycler. Anal Chem. 2004;76(14):4011–6.
Jiang L, Mancuso M, Lu Z, Akar G, Cesarman E, Erickson D. Solar thermal polymerase chain reaction for smartphone-assisted molecular diagnostics. Sci Rep. 2014;4:4137.
Hashimoto M, Chen P-C, Mitchell MW, Nikitopoulos DE, Soper SA, Murphy MC. Rapid PCR in a continuous flow device. Lab Chip. 2004;4(6):638–45.
Acknowledgments
The authors would like to thank Drs. S.E. Kakambakos and P.S. Petrou at IPRETEA, NCSR “Demokritos,” for providing access to their roll laminator.
Funding
This research was financially supported by the (1) FP7 “Love Wave Fully Integrated Lab-on-chip Platform for Food Pathogen Detection”—LOVE FOOD project (Contract No 317742)—and (2) Horizon 2020-EU 2.1.1, Project ID: 68768, “LOVEFOOD2Market—A portable MicroNanoBioSystem and Instrument for ultra-fast analysis of pathogens in food: Innovation from LOVE-FOOD lab prototype to a pre-commercial instrument” (http://lovefood2market.eu/).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaprou, G.D., Papadopoulos, V., Papageorgiou, D.P. et al. Ultrafast, low-power, PCB manufacturable, continuous-flow microdevice for DNA amplification. Anal Bioanal Chem 411, 5297–5307 (2019). https://doi.org/10.1007/s00216-019-01911-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01911-1