Abstract
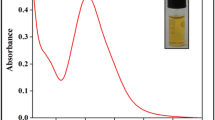
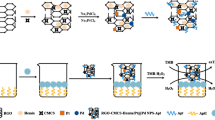
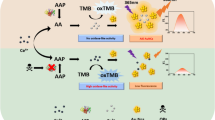
In this study, we developed a simple colorimetric approach to detect glutathione (GSH). The proposed approach is based on the ability of CuS-PDA-Au composite material to catalytically oxidize 3,3′,5,5′-tetramethylbenzidine (TMB) to ox-TMB to induce a blue color with an absorption peak centered at 652 nm. However, the introduction of GSH can result in a decrease in oxidized TMB; similarly, it can combine with Au nanoparticles (Au NPs) on the surface of CuS-PDA-Au composite material. Both approaches can result in a fading blue color and a reduction of the absorbance at 652 nm. Based on this above, we proposed a technique to detect GSH quantitatively and qualitatively through UV-Vis spectroscopy and naked eye, respectively. This approach demonstrates a low detection limit of 0.42 μM with a broad detection range of 5 × 10−7–1 × 10−4 M with the assistance of UV-Vis spectroscopy. More importantly, this approach is convenient and rapid. This method was successfully applied to GSH detection in human serum and cell lines.

A colorimetric approach has been developed by exploiting the peroxidase-like activity of CuS-polydopamine-Au composite for sensitive glutathione detection.





Similar content being viewed by others
References
Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263(33):17205–8.
Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Jr SP, Reed RL, et al. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24(5):699–704.
Pocernich CB, Butterfield DA. Elevation of glutathione as a therapeutic strategy in Alzheimer disease. Biochim Biophys Acta. 2012;1822(5):625–30.
Lu SC. Regulation of glutathione synthesis. Mol Asp Med. 2009;30(1–2):42–59.
Jimenez LA. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal. 2005;7(1–2):42–59.
Refsum H, Ueland PM, Nygård O, Vollset SE. Homocysteine and cardiovascular disease. Annu Rev Med. 1998;49(1):151–66.
Perry RR, Mazetta JA, Levin M, Barranco SC. Glutathione levels and variability in breast tumors and normal tissue. Cancer. 2015;72(3):783–7.
Xianyu Y, Xie Y, Wang N, Wang Z, Jiang X. A dispersion-dominated chromogenic strategy for colorimetric sensing of glutathione at the nanomolar level using gold nanoparticles. Small. 2015;11(41):5510–4.
Guo Y, Wang H, Sun Y, Qu B. A disulfide bound-molecular beacon as a fluorescent probe for the detection of reduced glutathione and its application in cells. Chem Commun. 2012;48(26):3221–3.
Zhang Q, Vakili MR, Li XF, Lavasanifar A, Le XC. Polymeric micelles for GSH-triggered delivery of arsenic species to cancer cells. Biomaterials. 2014;35(25):7088–100.
Feng J, Huang P, Shi S, Deng KY, Wu FY. Colorimetric detection of glutathione in cells based on peroxidase-like activity of gold nanoclusters: a promising powerful tool for identifying cancer cells. Anal Chim Acta. 2017;967:64–9.
Tcherkas YV, Denisenko AD. Simultaneous determination of several amino acids, including homocysteine, cysteine and glutamic acid, in human plasma by isocratic reversed-phase high-performance liquid chromatography with fluorimetric detection. J Chromatogr A. 2001;913(1):309–13.
Wang W, Li L, Liu S, Ma C, Zhang S. Determination of physiological thiols by electrochemical detection with piazselenole and its application in rat breast cancer cells 4T-1. J Am Chem Soc. 2008;130(33):10846–7.
Miao P, Liu L, Nie Y, Li G. An electrochemical sensing strategy for ultrasensitive detection of glutathione by using two gold electrodes and two complementary oligonucleotides. Biosens Bioelectron. 2009;24(11):3347–51.
Meng F, Miao P, Wang B, Tang Y, Yin J. Identification of glutathione by voltammetric analysis with rolling circle amplification. Anal Chim Acta. 2016;943:58–63.
Niu LY, Chen YZ, Zheng HR, Wu LZ, Tung CH, Yang QZ. ChemInform abstract: design strategies of fluorescent probes for selective detection among biothiols. Chem Soc Rev. 2015;46(42):6143–60.
Dai X, Du ZF, Wang LH, Miao JY, Zhao BX. A quick response fluorescent probe based on coumarin and quinone for glutathione and its application in living cells. Anal Chim Acta. 2016;922:64–70.
Rusin O, Luce NNS, Agbaria RA, Escobedo JO, Jiang S, Warner IM, et al. Visual detection of cysteine and Homocysteine. J Am Chem Soc. 2004;126(2):438–9.
Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1(6):3159–65.
Wei H, Wang E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42(14):6060–93.
Song Y, Qu K, Zhao C, Ren J, Qu X. Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater. 2010;22(19):2206–10.
Shamsipur M, Safavi A, Mohammadpour Z. Indirect colorimetric detection of glutathione based on its radical restoration ability using carbon nanodots as nanozymes. Sensors Actuators B Chem. 2014;199(4):463–9.
Wang Q, Zhang L, Shang C, Zhang Z, Dong S. Triple-enzyme mimetic activity of nickel-palladium hollow nanoparticles and their application in colorimetric biosensing of glucose. Chem Commun. 2016;52(31):5410–3.
Ge S, Liu W, Liu H, Liu F, Yu J, Yan M, et al. Colorimetric detection of the flux of hydrogen peroxide released from living cells based on the high peroxidase-like catalytic performance of porous PtPd nanorods. Biosens Bioelectron. 2015;71:456–62.
Dong Y, Zhang J, Jiang P, Wang G, Wu X, Zhao H, et al. Superior peroxidase mimetic activity of carbon dots–Pt nanocomposites relies on synergistic effects. New J Chem. 2015;39(5):4141–6.
Nasir M, Nawaz MH, Latif U, Yaqub M, Hayat A, Rahim A. An overview on enzyme-mimicking nanomaterials for use in electrochemical and optical assays. Microchim Acta. 2016:1–20.
Niu X, He Y, Pan J, Li X, Qiu F, Yan Y, et al. Uncapped nanobranch-based CuS clews used as an efficient peroxidase mimic enable the visual detection of hydrogen peroxide and glucose with fast response. Anal Chim Acta. 2016;947:42–9.
Zou H, Yang T, Lan J, Huang C. Use of the peroxidase mimetic activity of erythrocyte-like Cu1.8S nanoparticles in the colorimetric determination of glutathione. Anal Methods. 2017;9(5):841–6.
Qu K, Shi P, Ren J, Qu X. Nanocomposite incorporating V2O5 nanowires and gold nanoparticles for mimicking an enzyme cascade reaction and its application in the detection of biomolecules. Chemistry. 2014;20(24):7501–6.
Liang RP, Yao GH, Fan LX, Qiu JD. Magnetic Fe3O4@Au composite-enhanced surface plasmon resonance for ultrasensitive detection of magnetic nanoparticle-enriched α-fetoprotein. Anal Chim Acta. 2012;737(6):22–8.
Zhou H, Ning G, Li T, Cao Y, Zeng S, Lei Z, et al. The sandwich-type electrochemiluminescence immunosensor for α-fetoprotein based on enrichment by Fe3O4-Au magnetic nano probes and signal amplification by CdS-Au composite nanoparticles labeled anti-AFP. Anal Chim Acta. 2012;746:107–13.
Wang AJ, Ju KJ, Zhang QL, Song P, Wei J, Feng JJ. Folic acid bio-inspired route for facile synthesis of AuPt nanodendrites as enhanced electrocatalysts for methanol and ethanol oxidation reactions. J Power Sources. 2016;326:227–34.
Josephy PD, Eling T, Mason RP. The horseradish peroxidase-catalyzed oxidation of 3,5,3′,5′-tetramethylbenzidine. Free radical and charge-transfer complex intermediates. J Biol Chem. 1982;257(7):3669–75.
Bally RW, Gribnau TC. Some aspects of the chromogen 3,3′,5,5′-tetramethylbenzidine as hydrogen donor in a horseradish peroxidase assay. J Clin Chem Clin Biochem. 1989;27(10):791–6.
Yin J, Cao H, Lu Y. Self-assembly into magnetic Co3O4 complex nanostructures as peroxidase. J Mater Chem. 2011;22(2):527–34.
Liu M, Zhao H, Chen S, Yu H, Quan X. Interface engineering catalytic graphene for smart colorimetric biosensing. ACS Nano. 2012;6(4):3142–51.
Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577–83.
Yang XF, Huang Q, Zhong Y, Li Z, Li H, Lowry M, et al. A dual emission fluorescent probe enables simultaneous detection of glutathione and cysteine/homocysteine. Chem Sci. 2014;5(6):2177–83.
Yang W, Hao J, Zhang Z, Zhang B. PB@Co3O4nanoparticles as both oxidase and peroxidase mimics and their application for colorimetric detection of glutathione. New J Chem. 2015;39(11):8802–6.
Eyer P, Podhradský D. Evaluation of the micromethod for determination of glutathione using enzymatic cycling and Ellman's reagent. Anal Biochem. 1986;153(1):57–66.
Liu J, Meng L, Fei Z, Dyson PJ, Jing X, Liu X. MnO2 nanosheets as an artificial enzyme to mimic oxidase for rapid and sensitive detection of glutathione. Biosens Bioelectron. 2017;90:69–74.
Wang T, Su P, Li H, Yang Y, Yang Y. Triple-enzyme mimetic activity of Co3O4nanotubes and their applications in colorimetric sensing of glutathione. New J Chem. 2016;40(12):10056–63.
Ni P, Sun Y, Dai H, Hu J, Jiang S, Wang Y, et al. Highly sensitive and selective colorimetric detection of glutathione based on Ag [I] ion-3,3′,5,5′-tetramethylbenzidine (TMB). Biosens Bioelectron. 2015;63(2):47–52.
Zhang X, Mao X, Li S, Dong W, Huang Y. Tuning the oxidase mimics activity of manganese oxides via control of their growth conditions for highly sensitive detection of glutathione. Sensors Actuators B Chem. 2018;258:80–7.
Li S, Wang L, Zhang X, Chai H, Huang Y. A Co,N co-doped hierarchically porous carbon hybrid as a highly efficient oxidase mimetic for glutathione detection. Sensors Actuators B Chem. 2018;264:312–9.
Acknowledgments
We thank the anonymous reviewers for their valuable suggestions.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (21305097) and Two-way Support Plan Foundation of Sichuan Agricultural University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The project was approved by the Third People’s Hospital of Chengdu. All experiments were performed in compliance with the relevant laws and institutional guidelines.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 406 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, Y., Ding, F. et al. Colorimetric determination of glutathione in human serum and cell lines by exploiting the peroxidase-like activity of CuS-polydopamine-Au composite. Anal Bioanal Chem 410, 4805–4813 (2018). https://doi.org/10.1007/s00216-018-1117-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1117-4