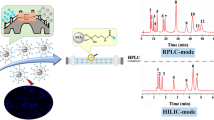
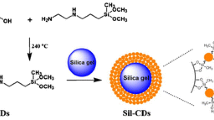
Abstract
In this paper, N-doped carbon dots (NCDs) were successfully decorated on the spherical porous silica surface in deep eutectic solvents (DESs) as a novel class of green solvents. The appropriate density and hydrophility of DESs guaranteed the fine dispersibility of silica particles and NCDs, resulting in a homogeneous and thin layer of NCDs immobilization. As compared with traditional organic solvents (DMF and THF), higher surface coverage was obtained in the medium of DES, proving its feasibility as a new kind of alternative solvent for hydrophilic nanomaterial-based surface modification of silica spheres. The resulting NCDs-decorated silica particles (Sil-NCDs) were characterized in detail and packed into chromatographic columns to study their initial feasibility as adsorbent material for liquid chromatography. The resultant packing materials demonstrate a selective behavior for polar compounds in hydrophilic interaction liquid chromatography (HILIC) mode. This work gives a typical example of using carbon dots as stationary phase component, and such material is hopeful to be used in other research fields such as solid absorbents, recycling catalysts, and solid-state electrochemistry etc.

N-doped carbon dots (NCDs) were successfully coupled on the surface of porous silica spheres in a green strategy using deep eutectic solvents (DES) as media for HILIC.








Similar content being viewed by others
References
Trojanowicz M. Analytical applications of carbon nanotubes: a review. TrAC Trends Anal Chem. 2006;25:480–9.
Ravelo-Pérez LM, Herrera-Herrera AV, Hernández-Borges J, Rodríguez-Delgado MÁ. Carbon nanotubes: solid-phase extraction. J Chromatogr A. 2010;1217:2618–41.
Hong G, Diao S, Antaris AL, Dai H. Carbon nanomaterials for biological imaging and nanomedicinal therapy. Chem Rev. 2015;115:10816–906.
Valcárcel M, Cárdenas S, Simonet BM. Role of carbon nanotubes in analytical science. Anal Chem. 2007;79:4788–97.
Speltini A, Merli D, Profumo A. Analytical application of carbon nanotubes, fullerenes and nanodiamonds in nanomaterials-based chromatographic stationary phases: a review. Anal Chim Acta. 2013;783:1–16.
Zhang M, Qiu H. Progress in stationary phases modified with carbonaceous nanomaterials for high-performance liquid chromatography. TrAC Trends Anal Chem. 2015;65:107–21.
Liu Q, Shi J, Sun J, Wang T, Zeng L, Jiang G. Graphene and graphene oxide sheets supported on silica as versatile and high-performance adsorbents for solid-phase extraction. Angew Chem Int Ed. 2011;50:5913–7.
Xue Z, Vinci JC, Colón LA. Nanodiamond-decorated silica spheres as a chromatographic material. ACS Appl Mater Interfaces. 2016;8:4149–57.
Cai T, Zhang H, Li Z, Rahman AFMM, Qiu H. A new nano-on-micro stationary phase based on nanodiamond bonded on silica for hydrophilic interaction chromatography. RSC Adv. 2016;6:32757–60.
Herrera-Herrera AV, González-Curbelo MÁ, Hernández-Borges J, Rodríguez-Delgado MÁ. Carbon nanotubes applications in separation science: a review. Anal Chim Acta. 2012;734:1–30.
Mukhtar NH, See HH. Carbonaceous nanomaterials immobilised mixed matrix membrane microextraction for the determination of polycyclic aromatic hydrocarbons in sewage pond water samples. Anal Chim Acta. 2016;931:57–63.
Baker SN, Baker GA. Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed. 2010;49:6726–44.
Wang Y, Zhuang Q, Ni Y. Facile microwave-assisted solid-phase synthesis of highly fluorescent nitrogen–sulfur-codoped carbon quantum dots for cellular imaging applications. Chem Eur J. 2015;21:13004–11.
Qu S, Zhou D, Li D, Ji W, Jing P, Han D, et al. Toward efficient orange emissive carbon nanodots through conjugated sp2-domain controlling and surface charges engineering. Adv Mater. 2016;28:3516–21.
Zhu X, Zhao T, Nie Z, Liu Y, Yao S. Non-redox modulated fluorescence strategy for sensitive and selective ascorbic acid detection with highly photoluminescent nitrogen-doped carbon nanoparticles via solid-state synthesis. Anal Chem. 2015;87:8524–30.
Zheng XT, Than A, Ananthanaraya A, Kim D-H, Chen P. Graphene quantum dots as universal fluorophores and their use in revealing regulated trafficking of insulin receptors in adipocytes. ACS Nano. 2013;7:6278–86.
Zhou J, Yang Y, Zhang C-y. A low-temperature solid-phase method to synthesize highly fluorescent carbon nitride dots with tunable emission. Chem Commun. 2013;49:8605–7.
Tang J, Zhang Y, Kong B, Wang Y, Da P, Li J, et al. Solar-driven photoelectrochemical probing of nanodot/nanowire/cell interface. Nano Lett. 2014;14:2702–8.
Wang F, Chen Y-h, Liu C-y, Ma D-g. White light-emitting devices based on carbon dots’ electroluminescence. Chem Commun. 2011;47:3502–4.
Wang Y, Kalytchuk S, Wang L, Zhovtiuk O, Cepe K, Zboril R, et al. Carbon dot hybrids with oligomeric silsesquioxane: solid-state luminophores with high photoluminescence quantum yield and applicability in white light emitting devices. Chem Commun. 2015;51:2950–3.
Wang F, Xie Z, Zhang H, Liu C-y, Zhang Y-g. Highly luminescent organosilane-functionalized carbon dots. Adv Funct Mater. 2011;21:1027–31.
Liu C, Bao L, Tang B, Zhao J-Y, Zhang Z-L, Xiong L-H, et al. Fluorescence-converging carbon nanodots-hybridized silica nanosphere. Small. 2016;12:4702–6.
Liu Y, Ma Y-j, Liu C-y, Zhang Z-y, Yang W-d, Nie S-d, et al. The effective removal of Cr(VI) ions by carbon dot-silica hybrids driven by visible light. RSC Adv. 2016;6:68530–7.
Qiu H, Jiang S, Liu X. N-Methylimidazolium anion-exchange stationary phase for high-performance liquid chromatography. J Chromatogr A. 2006;1103:265–70.
Shen A, Guo Z, Yu L, Cao L, Liang X. A novel zwitterionic HILIC stationary phase based on “thiol-ene” click chemistry between cysteine and vinyl silica. Chem Commun. 2011;47:4550–2.
Aral T, Aral H, Ziyadanoğulları B, Ziyadanoğulları R. Synthesis of a mixed-model stationary phase derived from glutamine for HPLC separation of structurally different biologically active compounds: HILIC and reversed-phase applications. Talanta. 2015;131:64–73.
Qiu H, Zhang M, Gu T, Takafuji M, Ihara H. A sulfonic-azobenzene-grafted silica amphiphilic material: a versatile stationary phase for mixed-mode chromatography. Chem Eur J. 2013;19:18004–10.
Lu J, Zhang W, Zhang Y, Zhao W, Hu K, Yu A, et al. A new stationary phase for high performance liquid chromatography: Calix[4]arene derivatized chitosan bonded silica gel. J Chromatogr A. 2014;1350:61–7.
Qiu H, Mallik AK, Sawada T, Takafuji M, Ihara H. New surface-confined ionic liquid stationary phases with enhanced chromatographic selectivity and stability by co-immobilization of polymerizable anion and cation pairs. Chem Commun. 2012;48:1299–301.
Ru KB. Low melting mixtures in organic synthesis—an alternative to ionic liquids? Green Chem. 2012;14:2969–82.
Carriazo D, Serrano MC, Gutierrez MC, Ferrer ML, del Monte F. Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem Soc Rev. 2012;41:4996–5014.
Tereshatov EE, Boltoeva MY, Folden CM. First evidence of metal transfer into hydrophobic deep eutectic and low-transition-temperature mixtures: indium extraction from hydrochloric and oxalic acids. Green Chem. 2016;18:4616–22.
Smith EL, Abbott AP, Ryder KS. Deep eutectic solvents (DESs) and their applications. Chem Rev. 2014;114:11060–82.
Nam MW, Zhao J, Lee MS, Jeong JH, Lee J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: application to flavonoid extraction from Flos sophorae. Green Chem. 2015;17:1718–27.
Liao H-G, Jiang Y-X, Zhou Z-Y, Chen S-P, Sun S-G. Shape-controlled synthesis of gold nanoparticles in deep eutectic solvents for studies of structure–functionality relationships in electrocatalysis. Angew Chem Int Ed. 2008;47:9100–3.
Wagle DV, Zhao H, Baker GA. Deep eutectic solvents: sustainable media for nanoscale and functional materials. Acc Chem Res. 2014;47:2299–308.
Querejeta-Fernández A, Hernández-Garrido JC, Yang H, Zhou Y, Varela A, Parras M, et al. Unknown aspects of self-assembly of PbS microscale superstructures. ACS Nano. 2012;6:3800–12.
Gu T, Zhang M, Chen J, Qiu H. A novel green approach for the chemical modification of silica particles based on deep eutectic solvents. Chem Commun. 2015;51:9825–8.
Zhang H, Chen Y, Liang M, Xu L, Qi S, Chen H, et al. Solid-phase synthesis of highly fluorescent nitrogen-doped carbon dots for sensitive and selective probing ferric ions in living cells. Anal Chem. 2014;86:9846–52.
Li Y, Xu L, Chen T, Liu X, Xu Z, Zhang H. Carbon nanoparticles from corn stalk soot and its novel application as stationary phase of hydrophilic interaction chromatography and per aqueous liquid chromatography. Anal Chim Acta. 2012;726:102–8.
Zhang M, Mallik AK, Takafuji M, Ihara H, Qiu H. Versatile ligands for high-performance liquid chromatography: an overview of ionic liquid-functionalized stationary phases. Anal Chim Acta. 2015;887:1–16.
Qiu H, Mallik AK, Takafuji M, Jiang S, Ihara H. New poly(ionic liquid)-grafted silica multi-mode stationary phase for anion-exchange/reversed-phase/hydrophilic interaction liquid chromatography. Analyst. 2012;137:2553–5.
Melnikov SM, Höltzel A, Seidel-Morgenstern A, Tallarek U. A molecular dynamics study on the partitioning mechanism in hydrophilic interaction chromatography. Angew Chem Int Ed. 2012;51:6251–4.
Hemström P, Irgum K. Hydrophilic interaction chromatography. J Sep Sci. 2006;29:1784–821.
Guo Y, Gaiki S. Retention and selectivity of stationary phases for hydrophilic interaction chromatography. J Chromatogr A. 2011;1218:5920–38.
Liang T, Fu Q, Shen A, Wang H, Jin Y, Xin H, et al. Preparation and chromatographic evaluation of a newly designed steviol glycoside modified-silica stationary phase in hydrophilic interaction liquid chromatography and reversed phase liquid chromatography. J Chromatogr A. 2015;1388:110–8.
Yang FQ, Li SP. Effects of sample preparation methods on the quantification of nucleosides in natural and cultured Cordyceps. J Pharm Biomed Anal. 2008;48:231–5.
Gong YX, Li SP, Li P, Liu JJ, Wang YT. Simultaneous determination of six main nucleosides and bases in natural and cultured Cordyceps by capillary electrophoresis. J Chromatogr A. 2004;1055:215–21.
Acknowledgements
Financial supports from the “Hundred Talents Program” of Chinese Academy of Sciences and the National Natural Science Foundation of China (21475142, 21611140105) and the funds for Distinguished Young Scientists of Gansu (1506RJDA281) and the top priority program of “One-Three-Five” Strategic Planning of Lanzhou Institute of Chemical Physics, CAS, are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 576 kb)
Rights and permissions
About this article
Cite this article
Zhang, H., Qiao, X., Cai, T. et al. Preparation and characterization of carbon dot-decorated silica stationary phase in deep eutectic solvents for hydrophilic interaction chromatography. Anal Bioanal Chem 409, 2401–2410 (2017). https://doi.org/10.1007/s00216-017-0187-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0187-z