Abstract
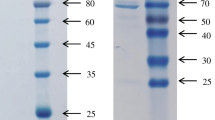
Organophosphorus pesticides (OPs) are the most widely used pesticides in agriculture, and OP residues have been broadly reported in food and environmental samples. The aim of this study is to develop a recombinant antibody-based broad-specificity immunoassay for OPs. A phage display library was prepared from a mouse pre-immunized with a generic immunogen of OPs, and a single-chain variable fragment (scFv) antibody was selected. The selected scFv antibody was fused with biotin acceptor domain (BAD) and overexpressed as an inclusion body in Escherichia coli BL21 (DE3). Then, the protein was refolded by stepwise urea gradient dialysis and biotinylated in vitro by E. coli biotin ligase (BirA). Subsequently, the scFv-BAD protein was purified from the biotinylated system with high yield (66.7 mg L−1) and confirmed by SDS-PAGE and Western blot. Based on the biotinylated scFv-BAD, a sensitive and broad-specificity competitive indirect enzyme-linked immunosorbent assay (ciELISA) for detection of OPs was developed. The cross-reactivity (CR) studies demonstrated that the ciELISA described here exhibited the broadest detection spectrum for OPs up to now, and 30 OPs could be determined with 50 % inhibition value (IC50) values ranging from 19.4 to 515.2 ng mL−1. Moreover, the developed ciELISA was used for the recovery study of the spiked samples and showed satisfactory recoveries.

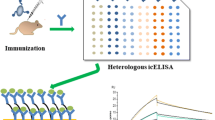
Schematic diagram of the development of biotinylated broad-specificity single-chain variable fragment antibody-based immunoassay for organophosphorus pesticides





Similar content being viewed by others
References
Liu DB, Chen WW, Wei JH, Li XB, Wang Z, Jiang XY. A highly sensitive, dual-readout assay based on gold nanoparticles for organophosphorus and carbamate pesticides. Anal Chem. 2012;84:4185–91.
Lu CS, Barr DB, Pearson MA, Waller LA. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect. 2008;116:537–42.
Palma P, Kuster M, Alvarenga P, Palma VL, Fernandes RM, Soares AMVM, et al. Risk assessment of representative and priority pesticides, in surface water of the Alqueva reservoir (South of Portugal) using on-line solid phase extraction-liquid chromatography-tandem mass spectrometry. Environ Int. 2009;35:545–51.
Long Q, Li HT, Zhang YY, Yao SZ. Upconversion nanoparticle-based fluorescence resonance energy transfer assay for organophosphorus pesticides. Biosens Bioelectron. 2015;68:168–74.
Yi YH, Zhu GB, Liu C, Huang Y, Zhang YY, Li HT, et al. A label-free silicon quantum dots-based photoluminescence sensor for ultrasensitive detection of pesticides. Anal Chem. 2013;85:11464–70.
Banks JN, Chaudhry MQ, Matthews WA, Haverly M, Watkins T, North Way BJ. Production and characterisation of polyclonal antibodies to the common moiety of some organophosphorus pesticides and development of a generic type ELISA. Food Agric Immunol. 1998;10:349–61.
Liang Y, Liu XJ, Liu Y, Yu XY, Fan MT. Synthesis of three haptens for the class-specific immunoassay of O,O-dimethyl organophosphorus pesticides and effect of hapten heterology on immunoassay sensitivity. Anal Chim Acta. 2008;615:174–83.
Li YL, Zhao FC, Zhao LY, Yang ZY. Development of a broad-specificity immunoassay for determination of organophosphorus pesticides using dual-generic hapten antigens. Food Anal Method. 2015;8:420–7.
Xu ZL, Xie GM, Li YX, Wang BF, Beier RC, Lei HT, et al. Production and characterization of a broad-specificity polyclonal antibody for O,O-diethyl organophosphorus pesticides and a quantitative structure–activity relationship study of antibody recognition. Anal Chim Acta. 2009;647:90–6.
Liu Y, Lou Y, Xu D, Qian GL, Zhang Q, Wu RR, et al. Production and characterization of monoclonal antibody for class-specific determination of O,O-dimethyl organophosphorus pesticides and effect of heterologous coating antigens on immunoassay sensitivity. Microchem J. 2009;93:36–42.
Xu ZL, Shen YD, Zheng WX, Beier RC, Xie GM, Dong JX, et al. Broad-specificity immunoassay for O,O-diethyl organophosphorus pesticides: application of molecular modeling to improve assay sensitivity and study antibody recognition. Anal Chem. 2010;82:9314–21.
Zhao FC, Hu CY, Wang HM, Zhao LY, Yang ZY. Development of a MAb-based immunoassay for the simultaneous determination of O,O-diethyl and O,O-dimethyl organophosphorus pesticides in vegetable and fruit samples pretreated with QuEChERS. Anal Bioanal Chem. 2015;407:8959–70.
Xu ZL, Dong JX, Wang H, Li ZF, Beier RC, Jiang YM, et al. Production and characterization of a single-chain variable fragment linked alkaline phosphatase fusion protein for detection of O,O-diethyl organophosphorus pesticides in a one-step enzyme-linked immunosorbent assay. J Agric Food Chem. 2012;60:5076–83.
Nishi N, Ishiuchi M, Morimune K, Ohkawa H. Molecular and immunochemical characteristics of monoclonal and recombinant antibodies selective for the triazine herbicide simetryn and application to environmental analysis. J Agric Food Chem. 2005;53:5096–104.
Plana E, Moreno MJ, Montoya Á, Manclús JJ. Development and application of recombinant antibody-based immunoassays to tetraconazole residue analysis in fruit juices. Anal Method. 2014;143:205–13.
Charlton K, Harris WJ, Porter AJ. The isolation of super-sensitive anti-hapten antibodies from combinatorial antibody libraries derived from sheep. Biosens Bioelectron. 2001;16:639–46.
Edupuganti SR, Edupuganti OP, O’Kennedy R. Generation of anti-zearalenone scFv and its incorporation into surface. Food Control. 2013;34:668–74.
Rangnoi K, Jaruseranee N, O’Kennedy R, Pansri P, Yamabhai M. One-step detection of Aflatoxin-B1 using scFv-alkaline phosphatase-fusion selected from human phage display antibody library. Mol Biotechnol. 2011;49:240–9.
Zhang XG, Xie JS, Sun Y, Xu HJ, Du TH, Liu ZX, et al. High-level expression, purification, and characterization of bifunctional ScFv-9R fusion protein. Appl Microbiol Biotechnol. 2014;98:5499–506.
Kudou M, Ejimab D, Satob H, Yumiokab R, Arakawac T, Tsumotoa K. Refolding single-chain antibody (scFv) using lauroyl-L-glutamate as a solubilization detergent and arginine as a refolding additive. Protein Expr Purif. 2011;77:68–74.
Umetsu M, Tsumoto K, Hara M, Ashish K, Goda S, Adschiri T, et al. How additives influence the refolding of immunoglobulin-folded proteins in a stepwise dialysis system. J Biol Chem. 2003;278:8979–87.
Min WK, Kim SG, Seo JH. Affinity maturation of single-chain variable fragment specific for aflatoxin B1 using yeast surface display. Food Chem. 2015;188:604–11.
Rajpal A, Beyaz N, Haber L, Cappuccilli G, Yee H, Bhatt RR, et al. A general method for greatly improving the affinity of antibodies by using combinatorial libraries. Proc Natl Acad Sci U S A. 2005;102:8466–71.
Thie H, Binius S, Schirrmann T, Hust M, Dübel S. Multimerization domains for antibody phage display and antibody production. New Biotechnol. 2009;26:314–21.
Cloutier SM, Couty S, Terskikh A, Marguerat L, Crivelli V, Pugnieres M, et al. Streptabody, a high avidity molecule made by tetramerization of in vivo biotinylated, phage display-selected scFv fragments on streptavidin. Mol Immunol. 2000;37:1067–77.
Li Y, Sousa R. Expression and purification of E. coli BirA biotin ligase for in vitro biotinylation. Protein Expr Purif. 2012;82:162–7.
Krebber A, Bornhauser S, Burmester J, Honegger A, Willuda J, Bosshard HR, et al. Reliable cloning of functional antibody variable domains from hybridomas and spleen cell repertoires employing a reengineered phage display system. J Immuno Methods. 1997;201:35–55.
Garet E, Cabado AG, Vieites JM, González-Fernández Á. Rapid isolation of single-chain antibodies by phage display technology directed against one of the most potent marine toxins: Palytoxin. Toxicon. 2010;55:1519–26.
Mak SK, Shan G, Lee HJ, Watanabe T, Stoutamire DW, Gee SJ, et al. Development of a class selective immunoassay for the type II pyrethroid insecticides. Anal Chim Acta. 2005;534:109–20.
Wilkowska A, Biziuk M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011;125:803–12.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant numbers 30972050 and 31271873) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures were performed according to the ethical standards of the institution or practice.
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Fengchun Zhao and Yuan Tian contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2878 kb)
Rights and permissions
About this article
Cite this article
Zhao, F., Tian, Y., Wang, H. et al. Development of a biotinylated broad-specificity single-chain variable fragment antibody and a sensitive immunoassay for detection of organophosphorus pesticides. Anal Bioanal Chem 408, 6423–6430 (2016). https://doi.org/10.1007/s00216-016-9760-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9760-0