Abstract
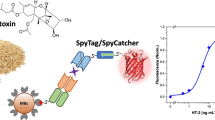
The recent development of a homogeneous time-resolved Förster resonance energy transfer (TR–FRET) immunoassay enables one-step, rapid (minutes), and direct detection compared to the multistep, time-consuming (hours), heterogeneous ELISA-type immunoassays. The use of the time-resolved effect of a donor lanthanide complex with a delay time of microseconds and large Stokes shift enables the separation of positive signals from the background autofluorescence of the sample. However, this study shows that the sample matrices directly interfere with donor fluorescence and that interference cannot be eliminated by time-resolved settings alone. Moreover, the reduction in donor emission did not appear to be equivalent to the reduction in acceptor emission, resulting in incorrect FRET signal measurements. To overcome this limitation, an internal standard approach was developed using an isotype control antibody. This new approach was used to develop TR–FRET assays for rapid detection (15–30 min) of Bacillus anthracis spores and botulinum toxin (type E) in beverages, which are major concerns in bioterrorism involving deliberate food contamination. Additionally, we demonstrate the detection of B. anthracis-secreted protective antigen (PA) and the Yersinia pestis-secreted markers F1 and LcrV in blood cultures, which are early markers of bacteremia in infected hosts following a possible bioterror attack. The use of an internal standard enables the calculation of correct ΔF values without the need for an external standard. Thus, the use of the internal standard approach in homogeneous immunoassays facilitates the examination of any sample regardless of its origin, and therefore expands the applicability of TR–FRET assays for complex matrices.






Similar content being viewed by others
References
Lee JS, Joung HA, Kim MG, Park CB. Graphene-based chemiluminescence resonance energy transfer for homogeneous immunoassay. ACS Nano. 2012;6(4):2978–83. doi:10.1021/nn300684d.
Watanabea J, Ishihara K. Single step diagnosis system using the FRET phenomenon induced by antibody-immobilized phosphorylcholine group-covered polymer nanoparticles. Sensors Actuators B Chem. 2008;129(1):87–93. doi:10.1016/j.snb.2007.07.100.
Wei Q, Lee M, Yu X, Lee EK, Seong GH, Choo J, et al. Development of an open sandwich fluoroimmunoassay based on fluorescence resonance energy transfer. Anal Biochem. 2006;358(1):31–7. doi:10.1016/j.ab.2006.08.019.
Boeneman K, Delehanty JB, Susumu K, Stewart MH, Deschamps JR, Medintz IL. Quantum dots and fluorescenct protein FRET-based biosensors. In: Zahavy E, Ordentlich A, Yitzhaki S, Shafferman A, editors. Nano-biotechnology for biomedical and diagnostic research. Advances in experimental medicine and biology. Dordrecht: Springer; 2012.
Hildebrandt N, Geissler D. Semiconductor Quntum dots as FRET acceptors for multiplexed diagnostic ruler application. In: Zahavy E, Ordentlich A, Yitzhaki S, Shafferman A, editors. Nano-biotechnology for biomedical and diagnostic research. Advances in experimental medicine and biology. Dordrecht: Springer; 2012.
Spindel S, Granek J, Sapsford KE. In vitro FRET sensing, diagnostic and personlized medicine. In: Medintz IL, Hildebrandt N, editors. FRET - from theory to application. Weinheim: Wiley-VCH; 2014.
Harma H, Dahne L, Pihlasalo S, Suojanen J, Peltonen J, Hanninen P. Sensitive quantitative protein concentration method using luminescent resonance energy transfer on a layer-by-layer europium(III) chelate particle sensor. Anal Chem. 2008;80(24):9781–6. doi:10.1021/ac801960c.
Kokko T, Liljenback T, Peltola MT, Kokko L, Soukka T. Homogeneous dual-parameter assay for prostate-specific antigen based on fluorescence resonance energy transfer. Anal Chem. 2008;80(24):9763–8. doi:10.1021/ac801875a.
Kupstat A, Kumke MU, Hildebrandt N. Toward sensitive, quantitative point-of-care testing (POCT) of protein markers: miniaturization of a homogeneous time-resolved fluoroimmunoassay for prostate-specific antigen detection. Analyst. 2011;136(5):1029–35. doi:10.1039/c0an00684j.
Medintz I, Hildebrandt N, editors. FRET - Förster resonance energy transfer: from theory to applications. Wiley Online Library; 2013.
Cohen N, Mechaly A, Mazor O, Fisher M, Zahavy E. Rapid homogenous time-resolved fluorescence (HTRF) immunoassay for anthrax detection. J Fluoresc. 2014;24(3):795–801. doi:10.1007/s10895-014-1354-7.
Geissler D, Stufler S, Lohmannsroben HG, Hildebrandt N. Six-color time-resolved Forster resonance energy transfer for ultrasensitive multiplexed biosensing. J Am Chem Soc. 2013;135(3):1102–9. doi:10.1021/ja310317n.
Mechaly A, Cohen N, Weiss S, Zahavy E. A novel homogeneous immunoassay for anthrax detection based on the AlphaLISA method: detection of B. anthracis spores and protective antigen (PA) in complex samples. Anal Bioanal Chem. 2013;405(12):3965–72. doi:10.1007/s00216-013-6752-1.
Qin QP, Peltola O, Pettersson K. Time-resolved fluorescence resonance energy transfer assay for point-of-care testing of urinary albumin. Clin Chem. 2003;49(7):1105–13.
Anisimov AP, Lindler LE, Pier GB. Intraspecific diversity of Yersinia pestis. Clin Microbiol Rev. 2004;17(2):434–64.
Skrzypek E, Straley SC. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J Bacteriol. 1995;177(9):2530–42.
Kobiler D, Weiss S, Levy H, Fisher M, Mechaly A, Pass A, et al. Protective antigen as a correlative marker for anthrax in animal models. Infect Immun. 2006;74(10):5871–6. doi:10.1128/IAI.00792-06.
Jernigan D, Raghunathan P, Bell B, Brechner R, Bresnitz E, Butler JC, et al. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg Infect Dis. 2002;8(10):1019–28. doi:10.3201/eid0810.020353.
Wein LM, Liu Y. Analyzing a bioterror attack on the food supply: the case of botulinum toxin in milk. Proc Natl Acad Sci U S A. 2005;102(28):9984–9. doi:10.1073/pnas.0408526102.
Cohen S, Mendelson I, Altboum Z, Kobiler D, Elhanany E, Bino T, et al. Attenuated nontoxinogenic and nonencapsulated recombinant Bacillus anthracis spore vaccines protect against anthrax. Infect Immun. 2000;68(8):4549–58.
Ben-Gurion R, Shafferman A. Essential virulence determinants of different Yersinia species are carried on a common plasmid. Plasmid. 1981;5(2):183–7.
Reuveny S, White MD, Adar YY, Kafri Y, Altboum Z, Gozes Y, et al. Search for correlates of protective immunity conferred by anthrax vaccine. Infect Immun. 2001;69(5):2888–93. doi:10.1128/IAI.69.5.2888-2893.2001.
Diamant E, Lachmi BE, Keren A, Barnea A, Marcus H, Cohen S, et al. Evaluating the synergistic neutralizing effect of anti-botulinum oligoclonal antibody preparations. PLoS One. 2014;9(1):e87089. doi:10.1371/journal.pone.0087089.
Levy Y, Flashner Y, Tidhar A, Zauberman A, Aftalion M, Lazar S, et al. T cells play an essential role in anti-F1 mediated rapid protection against bubonic plague. Vaccine. 2011;29(40):6866–73. doi:10.1016/j.vaccine.2011.07.059.
Zahavy E, Fisher M, Bromberg A, Olshevsky U. Detection of frequency resonance energy transfer pair on double-labeled microsphere and Bacillus anthracis spores by flow cytometry. Appl Environ Microbiol. 2003;69(4):2330–9.
Flashner Y, Fisher M, Tidhar A, Mechaly A, Gur D, Halperin G, et al. The search for early markers of plague: evidence for accumulation of soluble Yersinia pestis LcrV in bubonic and pneumonic mouse models of disease. FEMS Immunol Med Microbiol. 2010;59(2):197–206. doi:10.1111/j.1574-695X.2010.00687.x.
Knepp AM, Grunbeck A, Banerjee S, Sakmar TP, Huber T. Direct measurement of thermal stability of expressed CCR5 and stabilization by small molecule ligands. Biochemistry. 2011;50(4):502–11. doi:10.1021/bi101059w.
Vagima Y, Levy Y, Gur D, Tidhar A, Aftalion M, Abramovich H, et al. Early sensing of Yersinia pestis airway infection by bone marrow cells. Front Cell Infect Microbiol. 2012;2:143. doi:10.3389/fcimb.2012.00143.
Turro N, Ramamurthy V, Scaiano J. Modern molecular photochemistry of organic molecules. New York: Wiley Online Library; 2012.
Janzen TW, Thomas MC, Goji N, Shields MJ, Hahn KR, Amoako KK. Rapid detection method for Bacillus anthracis using a combination of multiplexed real-time PCR and pyrosequencing and its application for food biodefense. J Food Prot. 2015;78(2):355–61. doi:10.4315/0362-028X.JFP-14-216.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were performed in accordance with Israeli law and were approved by the Ethics Committee for Animal Experiments at the Israel Institute for Biological Research.
Conflict of interest
The authors state that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Cohen, N., Zahavy, E., Zichel, R. et al. An internal standard approach for homogeneous TR–FRET immunoassays facilitates the detection of bacteria, biomarkers, and toxins in complex matrices. Anal Bioanal Chem 408, 5179–5188 (2016). https://doi.org/10.1007/s00216-016-9602-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9602-0