Abstract
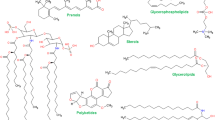
Lipid metabolic pathways play pivotal roles in liver function, and disturbances of these pathways are associated with various diseases. Thus, comprehensive characterization and measurement of lipid metabolites are essential to deciphering the contributions of lipid network metabolism to diseases or its responses to drug intervention. Here, we report an integrated lipidomic analysis for the comprehensive detection of lipid metabolites. To facilitate the characterization of untargeted lipids through fragmentation analysis, nine formulas were proposed to identify the fatty acid composition of lipids from complex MSn spectrum information. By these formulas, the co-eluted isomeric compounds could be distinguished. In total, 250 lipids were detected and characterized, including diacylglycerols, triacylglycerols, glycerophosphoethanolamines, glycerophosphocholines, glycerophosphoserines, glycerophosphoglycerols, glycerophosphoinositols, cardiolipins, ceramides, and sphingomyelins. Integrated with the targeted lipidomics, a total of 27 inflammatory oxylipins were also measured. To evaluate the aberrant lipid metabolism involved in liver injury induced by Tripterygium wilfordii, lipid network metabolism was further investigated. Results indicated that energy lipid modification, membrane remodeling, potential signaling lipid alterations, and abnormal inflammation response were associated with injury. Because of the important roles of lipids in liver metabolism, this new method is expected to be useful in analyzing other lipid metabolism diseases.






Similar content being viewed by others
References
Cajka T, Fiehn O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal Chem. 2015;88(1):524–45.
Brites P, Waterham HR, Wanders RJ. Functions and biosynthesis of plasmalogens in health and disease. Biochim Biophys Acta. 2004;1636(2–3):219–31.
Numata M, Chu HW, Dakhama A, Voelker DR. Pulmonary surfactant phosphatidylglycerol inhibits respiratory syncytial virus-induced inflammation and infection. Proc Natl Acad Sci U S A. 2010;107(1):320–5.
Chen S, Kong H, Lu X, Li Y, Yin P, Zeng Z, et al. Pseudotargeted metabolomics method and its application in serum biomarker discovery for hepatocellular carcinoma based on ultra high-performance liquid chromatography/triple quadrupole mass spectrometry. Anal Chem. 2013;85(17):8326–33.
Wolfer AM, Gaudin M, Taylor-Robinson SD, Holmes E, Nicholson JK. Development and validation of a high-throughput ultrahigh-performance liquid chromatography–mass spectrometry approach for screening of oxylipins and their precursors. Anal Chem. 2015;87(23):11721–31.
Garate J, Fernandez R, Lage S, Bestard-Escalas J, Lopez DH, Reigada R, et al. Imaging mass spectrometry increased resolution using 2-mercaptobenzothiazole and 2,5-diaminonaphthalene matrices: application to lipid distribution in human colon. Anal Bioanal Chem. 2015;407(16):4697–708.
Thomas A, Charbonneau JL, Fournaise E, Chaurand P. Sublimation of new matrix candidates for high spatial resolution imaging mass spectrometry of lipids: enhanced information in both positive and negative polarities after 1,5-diaminonapthalene deposition. Anal Chem. 2012;84(4):2048–54.
Kind T, Liu KH, Lee do Y, DeFelice B, Meissen JK, Fiehn O. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat Methods. 2013;10(8):755–8.
Narvaez-Rivas M, Zhang Q. Comprehensive untargeted lipidomic analysis using core-shell C30 particle column and high field orbitrap mass spectrometer. J Chromatogr A. 2016;1440:123–34.
Chen F, Ma YL, Ding H, Chen BP. Effects of Tripterygium wilfordii glycosides on regulatory T cells and Th17 in an IgA nephropathy rat model. Genet Mol Res. 2015;14(4):14900–7.
Li XJ, Jiang ZZ, Zhang LY. Triptolide: progress on research in pharmacodynamics and toxicology. J Ethnopharmacol. 2014;155(1):67–79.
Suciu M, Gruia AT, Nica DV, Azghadi SMR, Mic AA, Mic FA. Acetaminophen-induced liver injury: implications for temporal homeostasis of lipid metabolism and eicosanoid signaling pathway. Chem Biol Interact. 2015;242:335–44.
Cajka T, Fiehn O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trends Anal Chem. 2014;61:192–206.
Yang P, Chan D, Felix E, Madden T, Klein RD, Shureiqi I, et al. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins Leukot Essent Fat Acids. 2006;75(6):385–95.
Bird SS, Marur VR, Sniatynski MJ, Greenberg HK, Kristal BS. Serum lipidomics profiling using LC-MS and high-energy collisional dissociation fragmentation: focus on triglyceride detection and characterization. Anal Chem. 2011;83(17):6648–57.
Godzien J, Ciborowski M, Martínez-Alcázar MP, Samczuk P, Kretowski A, Barbas C. Rapid and reliable identification of phospholipids for untargeted metabolomics with LC–ESI–QTOF–MS/MS. J Proteome Res. 2015;14(8):3204–16.
Liebisch G, Vizcaino JA, Kofeler H, Trotzmuller M, Griffiths WJ, Schmitz G, et al. Shorthand notation for lipid structures derived from mass spectrometry. J Lipid Res. 2013;54(6):1523–30.
Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, et al. FAT SIGNALS—lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15(3):279–91.
Saponaro C, Gaggini M, Carli F, Gastaldelli A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients. 2015;7(11):9453–74.
Sapiro JM, Mashek MT, Greenberg AS, Mashek DG. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J Lipid Res. 2009;50(8):1621–9.
Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45(6):1366–74.
Mantzaris MD, Tsianos EV, Galaris D. Interruption of triacylglycerol synthesis in the endoplasmic reticulum is the initiating event for saturated fatty acid-induced lipotoxicity in liver cells. FEBS J. 2011;278(3):519–30.
Liu J, Han L, Zhu L, Yu Y. Free fatty acids, not triglycerides, are associated with non-alcoholic liver injury progression in high fat diet induced obese rats. Lipids Health Dis. 2016;15(1):27.
Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res. 2014;53:124–44.
Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281(17):12093–101.
Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, et al. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell. 2011;147(1):173–84.
Duwaerts CC, Maher JJ. Mechanisms of liver injury in non-alcoholic steatohepatitis. Curr Hepatol Rep. 2014;13(2):119–29.
Kusunoki N, Yamazaki R, Kitasato H, Beppu M, Aoki H, Kawai S. Triptolide, an active compound identified in a traditional Chinese herb, induces apoptosis of rheumatoid synovial fibroblasts. BMC Pharmacol. 2004;4:2.
Wang J, Miao M, Qu L, Cui Y, Zhang Y. Protective effects of geniposide against Tripterygium glycosides (TG)-induced liver injury and its mechanisms. J Toxicol Sci. 2016;41(1):165–73.
Yang X, Sheng W, Sun GY, Lee JC. Effects of fatty acid unsaturation numbers on membrane fluidity and alpha-secretase-dependent amyloid precursor protein processing. Neurochem Int. 2011;58(3):321–9.
Ariyama H, Kono N, Matsuda S, Inoue T, Arai H. Decrease in membrane phospholipid unsaturation induces unfolded protein response. J Biol Chem. 2010;285(29):22027–35.
Steenbergen R, Nanowski TS, Beigneux A, Kulinski A, Young SG, Vance JE. Disruption of the phosphatidylserine decarboxylase gene in mice causes embryonic lethality and mitochondrial defects. J Biol Chem. 2005;280(48):40032–40.
Dumas JF, Goupille C, Julienne CM, Pinault M, Chevalier S, Bougnoux P, et al. Efficiency of oxidative phosphorylation in liver mitochondria is decreased in a rat model of peritoneal carcinosis. J Hepatol. 2011;54(2):320–7.
Manganelli V, Capozzi A, Recalchi S, Signore M, Mattei V, Garofalo T, et al. Altered traffic of cardiolipin during apoptosis: exposure on the cell surface as a trigger for “antiphospholipid antibodies”. J Immunol Res. 2015;2015:847985.
Dumas JF, Peyta L, Couet C, Servais S. Implication of liver cardiolipins in mitochondrial energy metabolism disorder in cancer cachexia. Biochimie. 2013;95(1):27–32.
Patrussi L, Mariggio S, Corda D, Baldari CT. The glycerophosphoinositols: from lipid metabolites to modulators of T-cell signaling. Front Immunol. 2013;4:213.
Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–50.
Rodemer C, Thai TP, Brugger B, Kaercher T, Werner H, Nave KA, et al. Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice. Hum Mol Genet. 2003;12(15):1881–95.
Berger J, Dorninger F, Forss-Petter S, Kunze M. Peroxisomes in brain development and function. Biochim Biophys Acta. 2015;S0167–4889(15):00426–7.
Acknowledgments
This study was financially supported by the Natural Science Foundation of China (Grants No. 81303295, 81373688, 81573869); the Natural Science Foundation of Jiangsu Province, China (Grant No. BK20130959); The Specialized Research Fund for the Doctoral Program of Higher Education of China (Grant No. 20133237120001); colleges and universities in Jiangsu Province Natural Science Research (Grant No. 13KJB360005), and the Open Project Program of Jiangsu Key Laboratory of Pediatric Respiratory Disease, Nanjing University of Chinese Medicine (Grant No. JKLPRD201407).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Animal experiments were carried out in compliance with the standard ethical guidelines and under the control of the university ethical committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 969 kb)
Rights and permissions
About this article
Cite this article
Xie, T., Zhou, X., Wang, S. et al. Development and application of a comprehensive lipidomic analysis to investigate Tripterygium wilfordii-induced liver injury. Anal Bioanal Chem 408, 4341–4355 (2016). https://doi.org/10.1007/s00216-016-9533-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9533-9