Abstract
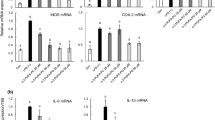
Diarylheptanoid A, 5-hydroxy-7-(4′-hydroxy-3′-methoxyphenyl)-1-phenyl-3-heptanone, is a naturally occurring phytochemical ingredient isolated from the rhizome of Alpinia officinarum. In order to confirm the anti-inflammatory activity of diphenylheptane A, we investigated its effects on lipopolysaccharide (LPS)-induced pro-inflammatory mediators, such as nitric oxide (NO), prostaglandin E2 (PGE2), interleukin-1β (IL-1β), and tumor necrosis factor α (TNF-α), as well as upstream genes, including the inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), nuclear factor κB (NF-κB) p65, p38 mitogen-activated protein kinase (MAPK), and extracellular signal-regulated kinase 1/2 (ERK1/2). Our results have proved the anti-inflammatory property of diphenylheptane A. Based on this finding, an LPS-induced RAW264.7 cell inflammatory model was introduced to evaluate the anti-inflammatory activity associated with glycerophospholipid (GPL) metabolism regulated by diphenylheptane A. We applied ultra-performance liquid chromatography/electrospray ionization-quadruple time of flight-mass spectrometry (UPLC/ESI-QTOF-MS) to the metabolic profiling of GPL synthesis in LPS-stimulated macrophages with the aim of identifying differentially synthesized GPL metabolites. Sixteen GPL metabolites, whose changes were restored to normal level after diphenylheptane A treatment, were further screened to be considered as useful biomarkers of inflammation. Overall, our study revealed for the first time that diphenylheptane A reestablished the production of 16 plasma membrane GPLs to basal level in LPS-activated RAW264.7 cells, suggesting the potential therapeutic property of phytochemical compounds against inflammatory diseases.

The schematic diagram of the study











Similar content being viewed by others
References
Ly TN, Shimoyamada M, Kato K, Yamauchi R. Isolation and characterization of some antioxidative compounds from the rhizomes of smaller galanga (Alpinia officinarum Hance). J Agric Food Chem. 2003;51:4924–9.
Ly TN, Yamauchi R, Shimoyamada M, Kato K. Isolation and structural elucidation of some glycosides from the rhizomes of smaller galanga (Alpinia officinarum Hance). J Agric Food Chem. 2002;50:4919–24.
Miller KI, Qing C, Sze DMY, Neilan BA. Investigation of the biosynthetic potential of endophytes in traditional Chinese anticancer herbs. Plos One. 2012;7:e35953.
Lin LY, Peng CC, Yeh XY, Huang BY, Wang HE, Chen KC, et al. Antihyperlipidemic bioactivity of Alpinia officinarum (Hance) Farw Zingiberaceae can be attributed to the coexistence of curcumin, polyphenolics, dietary fibers and phytosterols. Food Funct. 2015;6:1600–10.
Köse LP, Gülçin İ, Gören AC, Namiesnik J, Martinez-Ayala AL, Gorinstein S. LC–MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind Crop Prod. 2015;74:712–21.
Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–5.
Lee J, Kim KA, Jeong S, Lee S, Park HJ, Kim NJ, et al. Anti-inflammatory, anti-nociceptive, and anti-psychiatric effects by the rhizomes of Alpinia officinarum on complete Freund’s adjuvant-induced arthritis in rats. J Ethnopharmacol. 2009;126:258–64.
Yadav PN, Liu Z, Rafi MM. A diarylheptanoid from lesser galangal (Alpinia officinarum) inhibits proinflammatory mediators via inhibition of mitogen-activated protein kinase, p44/42, and transcription factor nuclear factor-kappa B. J Pharmacol Exp Ther. 2003;305:925–31.
Alhouayek M, Muccioli GG. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci. 2014;35:284–92.
Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002;2:787–95.
Chen GY, Nuňez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–37.
Eigler A, Moeller J, Endres S. Exogenous and endogenous nitric oxide attenuates tumor necrosis factor synthesis in the murine macrophage cell line RAW 264.7. J Immunol. 1995;154:4048–54.
Norris PC, Dennis EA. A lipidomic perspective on inflammatory macrophage eicosanoid signaling. Adv Biol Regul. 2014;54:99–110.
Hsu HY, Wen MH. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002;277:22131–9.
Lantz RC, Chen GJ, Sarihan M, Sólyom AM, Jolad SD, Timmermann BN. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine. 2007;14:123–8.
Brouwers JF. Liquid chromatographic–mass spectrometric analysis of phospholipids. chromatography, ionization and quantification. Biochim Biophys Acta. 2011;1811:763–75.
Watson AD. Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006;47:2101–11.
Khalil MB, Hou W, Zhou H, Elisma F, Swayne LA, Blanchard AP, et al. Lipidomics era: accomplishments and challenges. Mass Spectrom Rev. 2010;29:877–929.
Astudillo AM, Balgoma D, Balboa MA, Balsinde J. Dynamics of arachidonic acid mobilization by inflammatory cells. Biochim Biophys Acta. 2012;1821:249–56.
Andreyev AY, Fahy E, Guan Z, Kelly S, Li X, McDonald JG, et al. Subcellular organelle lipidomics in TLR-4-activated macrophages. J Lipid Res. 2012;51:2785–97.
Krauss RM. Phospholipid transfer protein and atherosclerosis: genetic studies take aim at a moving target. Circulation. 2010;122:452–4.
Zhu C, Liang QL, Hu P, Wang YM, Luo GA. Phospholipidomic identification of potential plasma biomarkers associated with type 2 diabetes mellitus and diabetic nephropathy. Talanta. 2011;85:1711–20.
Igarashi M, Ma K, Gao F, Kim HW, Rapoport SI, Rao JS. Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer’s disease prefrontal cortex. J Alzheimers Dis. 2011;24:507–17.
Jeong RU, Lim S, Kim MO, Moon MH. Effect of D-allose on prostate cancer cell lines: phospholipid profiling by nanoflow liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401:689–98.
Schmerler D, Neuqebauer S, Ludewig K, Bremer-Streck S, Brunkhorst FM, Kiehntopf M. Targeted metabolomics for discrimination of systemic inflammatory disorders in critically ill patients. J Lipid Res. 2012;53:1369–75.
Fitzpatrick FA, Soberman R. Regulated formation of eicosanoids. J Clin Invest. 2001;107:1347–51.
Kiuchi F, Iwakami S, Shibuya M, Hanaoka F, Sankawa U. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptanoids. Chem Pharm Bull (Tokyo). 1992;40:387–91.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
Miles AM, Wink DA, Cook JC, Grisham MB. Determination of nitric oxide using fluorescence spectroscopy. Methods Enzymol. 1996;268:105–20.
Ivanova PT, Milne SB, Byrne MO, Xiang Y, Brown HA. Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry. Methods Enzymol. 2007;432:21–57.
Wu X, Zhao LL, Peng HB, She YQ, Feng YF. Search for potential biomarkers by UPLC/Q-TOF-MS analysis of dynamic changes of glycerophospholipid constituents of RAW264.7 cells treated with NSAID. Chromatographia. 2015;78:211–20.
Pi JJ, Wu X, Yang ST, Zeng PY, Feng YF. Rapid identification of erythrocyte phospholipids in Sprague–Dawley rats by ultra high performance liquid chromatography with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Sep Sci. 2015;38:886–93.
Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res. 2001;480–481:243–68.
D’Acquisto F, Iuvone T, Rombolà L, Sautebin L, Di Rosa M, Carnuccio R. Involvement of NF-κB in the regulation of cyclooxygenase-2 protein expression in LPS-stimulated J774 macrophages. FEBS Lett. 1997;418:175–8.
Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–8.
Dilshara MG, Lee KT, Kim HJ, Lee HJ, Choi YH, Lee CM, et al. Anti-inflammatory mechanism of a-viniferin regulates lipopolysaccharide-induced release of proinflammatory mediators in BV2 microglial cells. Cell Immunol. 2014;290:21–9.
She YQ, Song JA, Yang E, Zhao LL, Zhong YM, Rui W, et al. Rapid identification of glycerophospholipids from RAW264.7 cells by UPLC/ESI-QTOF-MS. Biomed Chromatogr. 2014;28:1744–55.
Lichtenberger LM, Zhou Y, Jayaraman V, Doyen JR, O’Neil RG, Dial EJ, et al. Insight into NSAID-induced membrane alterations, pathogenesis and therapeutics: characterization of interaction of NSAIDs with phosphatidylcholine. Bichim Biophys Acta. 2012;1821:994–1002.
Liu ZH, Sang SM, Hartman TG, Ho CT, Rosen RT. Determination of diarylheptanoids from Alpinia officinarum (Lesser galangal) by HPLC with photodiode array and electrochemical detection. Phytochem Anal. 2005;16:252–6.
Yasukawa K, Sun Y, Kitanaka S, Tomizawa N, Miura M, Motohashi S. Inhibitory effect of the rhizomes of Alpinia officinarum on TPA-induced inflammation and tumor promotion in two-stage carcinogenesis in mouse skin. J Nat Med. 2008;62:374–8.
Matsuda H, Ando S, Kato T, Morikawa T, Yoshikawa M. Inhibitors from the rhizomes of Alpinia officinarum on production of nitric oxide in lipopolysaccharide-activated macrophages and the structural requirements of diarylheptanoids for the activity. Bioorg Med Chem. 2006;14:138–42.
Lee HJ, Kim JS, Ryu JH. Suppression of inducible nitric oxide synthase expression by diarylheptanoids from Alpinia officinarum. Planta Med. 2006;72:68–71.
Nakagawa Y, Waku K. The metabolism of glycerophospholipid and its regulation in monocytes and macrophages. Prog Lipid Res. 1989;28:205–43.
Lee SH, Soyoola E, Moncada S. Influence of cholesterol feeding on the production of eicosanoids, tissue plasminogen activator and superoxide anion (O2−) by rabbit blood monocytes. Atherosclerosis. 1986;61:141–8.
Pontsler AV, Hilaire AS, Marathe GK, Zimmerman GA, McIntyre TM. Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor gamma and oxidized alkyl phospholipids from oxidized low density lipoprotein. J Biol Chem. 2002;277:13029–36.
Kiuchi F, Shibuya M, Sankawa U. Inhibitors of prostaglandin biosynthesis from Alpinia officinarum. Chem Pharm Bull (Tokyo). 1982;30:2279–82.
Kim YI, Park SW, Kang IJ, Shin MK, Lee MH. Activin suppresses LPS-induced Toll-like receptor, cytokine and inducible nitric oxide synthase expression in normal human melanocytes by inhibiting NF-κB and MAPK pathway activation. Int J Mol Med. 2015;36:1165–72.
Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–92.
Zhou YQ, Chen YQ, Fisher JH, Wang MH. Activation of the RON receptor tyrosine kinase by macrophage-stimulating protein inhibits inducible cyclooxygenase-2 expression in murine macrophages. J Biol Chem. 2002;277:38104–10.
Chen CC, Wang JK. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 macrophages. Mol Pharmacol. 1999;55:481–8.
Wang T, Zhang X, Li JJ. The role of NF-B in the regulation of cell stress responses. Int Immuno Pharmacol. 2002;2:1509–20.
Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–5.
Calder PC, Yaqoob P, Thies F, Wallace FA, Miles EA. Fatty acids and lymphocyte functions. Br J Nutr. 2002;87:S31–48.
Zhang TT, Chen S, Liang XL, Zhang H. Development of a mass-spectrometry-based lipidomics platform for the profiling of phospholipids and sphingolipids in brain tissues. Anal Bioanal Chem. 2015;407:6543–55.
Karlsson AÂ, Michelsen P, Larsen Â, Odham G. Normal-phase liquid chromatography class separation and species determination of phospholipids utilizing electrospray mass spectrometry/tandem mass spectrometry. Rapid Commun Mass Spectrom. 1996;10:775–80.
Smith JC, Hou W, Whitehead SN, Ethier M, Bennett SAL, Daniel F. Identification of lysophosphatidylcholine (LPC) and platelet activating factor (PAF) from PC12 cells and mouse cortex using liquid chromatography/multi-stage mass spectrometry (LC/MS3). Rapid Commun Mass Spectrom. 2008;22:3579–87.
Wu X, Li Y, Wang Q, Li W, Feng Y. Effects of berberine and pomegranate seed oil on plasma phospholipid metabolites associated with risks of type 2 diabetes mellitus by U-HPLC/Q-TOF-MS. J Chromatogr B. 2015;1007:110–20.
Cordeiro FB, Cataldi TR, Perkel KJ, da Costa do Vale Teixeira L, Rochetti RC, Stevanato J, et al. Lipidomics analysis of follicular fluid by ESI-MS reveals potential biomarkers for ovarian endometriosis. Jassist Reprod Genet. 2015;32:1817–25.
Fazlollahi F, Kongmanas K, Tanphaichitr N, Suh J, Faull K, Gopen Q. Lipidomic profiling of mastoid bone and tissue from patients with chronic otomastoiditis. Int Arch Otorhinolaryngol. 2015;19:141–50.
Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res. 2004;43:3–35.
Rouzer CA, Ivanova PT, Byrne MO, Brown HA, Marnett LJ. Lipid profiling reveals glycerophospholipid remodeling in zymosan-stimulated macrophages. Biochemistry. 2007;46:6026–42.
Serhan CN, Clish CB, Btannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with anti-inflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–204.
Acknowledgments
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (NSFC, Grant Nos. 21275036 and 81202429).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by the National Natural Science Foundation of China (NSFC, Grant Nos. 21275036 and 81202429).
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 430 kb)
Rights and permissions
About this article
Cite this article
She, Y., Zheng, Q., Xiao, X. et al. An analysis on the suppression of NO and PGE2 by diphenylheptane A and its effect on glycerophospholipids of lipopolysaccharide-induced RAW264.7 cells with UPLC/ESI-QTOF-MS. Anal Bioanal Chem 408, 3185–3201 (2016). https://doi.org/10.1007/s00216-016-9383-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9383-5