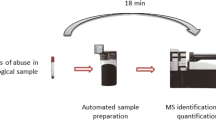
Abstract
We report the use of auto-sampler programmable functions to co-inject analyte standard solution and matrix extract to assess ion enhancement and suppression (matrix effects) in LC-MS. This is effectively an automated post-extraction addition (APEA) procedure, emulating the manual post-extraction addition (PEA) approach widely adopted for assessment of matrix effects. To verify that APEA was comparable to the conventional PEA approach, matrix effects were determined using both methods for a selection of 31 illicit and pharmaceutical drugs in 10 different human urine extracts. Matrix effects measured using APEA were statistically indistinguishable from manual PEA methodology for 27 of the 31 drugs. Of the four drugs that showed significant differences using the two methods, three differed by less than 2 %, which is within the expected accuracy limits required for matrix effect determinations. The remaining analyte, trimeprazine, was found to degrade in the spiked PEA matrix extract, accounting for the difference between matrix effects measured by the PEA and APEA approaches. APEA enables a single matrix extract to be assessed at multiple analyte concentrations, resulting in a considerable reduction in sample preparation time. In addition, APEA can reduce the quantity of analyte-free sample matrix required for matrix effect assessment, which is an important consideration in certain analytical and bioanalytical fields. This work shows that APEA may be considered as an acceptable alternative to PEA for the assessment of matrix effects in LC-MS method validation and may be applicable to a variety of matrices such as environmental samples.

A graphical representation of the procedure used to compare manual post extraction addition (PEA) and auto-sampler programmed co-injection (APEA) to assess matrix effects in LCMS


Similar content being viewed by others
References
Annesley TM. Ion suppression in mass spectrometry. Clin Chem. 2003;49:1041–4.
Taylor PJ. Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry. Clin Biochem. 2005;38:328–34.
Kruve A, Leito I. Comparison of different methods aiming to account for/overcome matrix effects in LC/ESI/MS on the example if pesticide analyses. Anal Methods. 2013;5:3035–44.
Van Eeckhaut A, Lanckmans K, Sarre S, Smolders I, Michotte Y. Validation of bioanalytical LC-MS/MS assays: evaluation of matrix effects. J Chromatogr B. 2009;887:2198–207.
Trufelli H, Palma P, Famiglini G, Cappiello A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom Rev. 2011;30:491–509.
Stahnke H, Reemtsma T, Alder L. Compensation of matrix effects by post-column infusion of a monitor substance in multiresidue analysis with LC-MS/MS. Anal Chem. 2009;81:2185–92.
Butzbach DM. The influence of putrefaction and sample storage in post-mortem toxicology results. Forensic Sci Med Pathol. 2010;6:35–45.
Peters FT, Drummer OH, Musshoff F. Validation of new methods. Forensic Sci Int. 2007;165:216–24.
Maurer HH. Current role of liquid chromatography–mass spectrometry in clinical and forensic toxicology. Anal Bioanal Chem. 2007;388:1315–25.
Matuszewski BK, Constanzer ML, Chavez-Eng CM. Matrix effect in quantitative LC/MS/MS analyses of biological fluids: a method for determination of finasteride in human plasma at picogram per milliliter concentrations. Anal Chem. 1998;70:882–9.
Peters FT. Recent advances of liquid chromatography-(tandem) mass spectrometry in clinical and forensic toxicology. Clin Biochem. 2011;44:54–65.
Peters FT, Remane D. Aspects of matrix effects in applications of liquid chromatography-mass spectrometry to forensic and clinical toxicology—a review. Anal Bioanal Chem. 2012;403:2155–72.
National Association of Testing Authorities (2004) Technical note 17: Guidelines for the validation and verification of quantitative and qualitative test methods. Available at: http://www.nata.com.au/nata/phocadownload/publications/Guidance_information/tech-notes-information-papers/technical_note_17.pdf. Accessed 27 July 2015.
Committee for Medicinal Products for Human Use (2011) European Medicines Agency. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. Accessed 27 July 2015
Scientific Working Group for Forensic Toxicology. Standard practices for method validation in forensic toxicology. J Anal Toxicol. 2013;37:452–74.
Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, et al. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm Res. 2007;24:1962–73.
Peters FT, Hartung M, Schmitt MHG, Daldrup T, Musshoff F. Requirements for the validation of analytical methods. Toxichem Krimtech. 2009;76:185–208.
Bonfiglio R, King RC, Olah TV, Merkle K. The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun Mass Spectrom. 1999;13:1175–85.
Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–30.
Saar E, Gerostamoulos D, Drummer OH. Comparison of extraction efficiencies and LC-MS-MS matrix effects using LLE and SPE methods for 19 antipsychotics in human blood. Anal Bioanal Chem. 2009;393:727–34.
Kostakis C, Harpas P, Stockham P. Forensic toxicology. In: Fanali S, Haddad PR, Poole CF, Schoenmakers P, Lloyd D, editors. Liquid chromatography: applications. Waltham: Elsevier; 2013. p. 249–94.
Acknowledgments
The authors thank Forensic Science South Australia for providing funding for this research through the Summer Student Scholarship program, and the Toxicology Department at Forensic Science SA for the use of equipment. The authors acknowledge and thank Agilent Technologies for a funding of a summer scholarship in support of these studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Biological samples used in this work were collected with ethical consideration and under SAC HREC where appropriate.
Conflict of interest
Caitlyn Rogers was supported with a summer studentship from Forensic Science South Australia, Sheridan Martin was supported with a summer studentship from Agilent Technologies. There are no other financial or non-financial interests to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 91 kb)
Rights and permissions
About this article
Cite this article
Rogers, C.A., Stockham, P.C., Nash, C.M. et al. An alternative approach for assessment of liquid chromatography-mass spectrometry matrix effects using auto-sampler programmed co-injection. Anal Bioanal Chem 408, 2009–2017 (2016). https://doi.org/10.1007/s00216-015-9278-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9278-x