Abstract
Platelet activation is a key process in blood clot formation. During activation, platelets go through both chemical and physical changes, including secretion of chemical messengers and cellular shape change. Platelet shape change is mediated by the two major cytoskeletal elements in platelets, the actin matrix and microtubule ring. Most studies to date have evaluated these structures qualitatively, whereas this paper aims to provide a quantitative method of examining changes in these structures by fluorescently labeling the element of interest and performing single cell image analysis. The method described herein tracks the diameter of the microtubule ring and the circumference of the actin matrix as they change over time. Platelets were incubated with a series of drugs that interact with tubulin or actin, and the platelets were observed for variation in shape change dynamics throughout the activation process. Differences in shape change mechanics due to drug incubation were observable in each case.

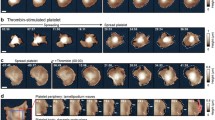
Cytoskeleton rearrangement upon platelet activation. Platelet elements are colored as follows: orange - microtubule ring, red - actin matrix, green - dense-body granules.





Similar content being viewed by others
References
Golebiewska EM, Poole AW (2014) Secrets of platelet exocytosis - what do we really know about platelet secretion mechanisms? Br J Haematol 165:204–216
Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE (2014) Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol 229:1005–1015
Mannaioni PF, Di Bello MG, Masini E (1997) Platelets and inflammation: role of platelet-derived growth factor, adhesion molecules and histamine. Inflamm Res 46:4–18
Rendu F, Brohard-Bohn B (2001) The platelet release reaction: granules’ constituents, secretion and functions. Platelets 12:261–273
Blockmans D, Deckmyn H, Vermylen J (1995) Platelet activation. Blood Rev 9:143–156
Ge S, White JG, Haynes CL (2010) Critical role of membrane cholesterol in exocytosis revealed by single platelet study. ACS Chem Biol 5:819–828
Ge S, White JG, Haynes CL (2012) Cytoskeletal F-actin, not the circumferential coil of microtubules, regulates platelet dense-body granule secretion. Platelets 23:259–263
Koseoglu S, Dilks JR, Peters CG, Fitch-Tewfik JL, Fadel NA, Jasuja R, Italiano JE, Haynes CL, Flaumenhaft R (2013) Dynamin-related protein-1 controls fusion pore dynamics during platelet granule exocytosis. Arterioscler Thromb Vasc Biol 33:481–488
Ge S, Woo E, White JG, Haynes CL (2011) Electrochemical measurements of endogenous serotonin release from human blood platelets. Anal Chem 83:2598–2604
Ge S, White JG, Haynes CL (2009) Quantal release of serotonin from platelets. Anal Chem 81:2935–2943
Ge S, Wittenberg NJ, Haynes CL (2008) Quantitative and real-time detection of chemical messenger secretion from platelets. Biochemistry 47:7020–7024
Severin S, Gaits-Iacovoni F, Allart S, Gratacap MP, Payrastre B (2013) A confocal-based morphometric analysis shows a functional crosstalk between the actin filament system and microtubules in thrombin-stimulated platelets. J Thromb Haemost 11:183–216
Patel-Hett S, Richardson JL, Schulze H, Drabek K, Isaac NA, Hoffmeister K, Shivdasani RA, Bulinski JC, Galjart N, Hartwig J, Italiano JE (2008) Visualization of microtubule growth in living platelets reveals a dynamic marginal band with multiple microtubules. Blood 111:4605–4616
Hartwig J, Barkalow K, Azim A, Italiano JE (1999) The elegant platelet: signals controlling actin assembly. Thromb Haemost 82:392–398
Gear ARL, Burke D (1982) Thrombin-induced secretion of serotonin from platelets can occur in seconds. Blood 60:1231–1234
Cerecedo D, Stock R, Gonzalez S, Reyes E, Mondragon R (2002) Modification of actin, myosin and tubulin distribution during cytoplasmic granule movement associated with platelet adhesion. Haematologica 87:1165–1176
Asmis L, Tanner FC, Sudano I, Lüscher TF, Camici GG (2010) DMSO inhibits human platelet activation through cyclooxygenase-1 inhibition. A novel agent for drug eluting stents? Biochem Biophys Res Commun 391:1629–1633
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265
Coue M, Brenner SL, Spector I, Korn ED (1987) Inhibition of actin polymerization by latrunculin A. FEBS Lett 213:316–318
Cooper JA (1987) Effects of cytochalasin and phalloidin on actin. J Cell Biol 105:1473–1478
Cerecedo D, Cisneros B, Mondragon R, Gonzalez S, Galván IJ (2010) Actin filaments and microtubule dual-granule transport in human adhered platelets: the role of α-dystrobrevins. Br J Haematol 149:124–136
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 325 kb)
Rights and permissions
About this article
Cite this article
Finkenstaedt-Quinn, S.A., Ge, S. & Haynes, C.L. Cytoskeleton dynamics in drug-treated platelets. Anal Bioanal Chem 407, 2803–2809 (2015). https://doi.org/10.1007/s00216-015-8523-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8523-7