Abstract
This paper describes a new method for the determination of polar triazines, including some degradation products, which combines online in-tube solid-phase microextraction (IT-SPME) and capillary liquid chromatography with UV-diode array detection (DAD). Different extractive coatings have been evaluated for IT-SPME, a capillary column with a polydimethylsiloxane (PDMS) coating, and the same coating modified with carboxylated single-wall carbon nanotubes (c-SWCNTs) and carboxylated multiwall carbon nanotubes (c-MWCNTs). On the basis of the results obtained, a new method is presented for the identification and determination of triazines in water samples. A careful selection of the eluent composition provides the required selectivity and sensitivity for the quantification of the target analytes, even those highly polar (log K ow ≤ 2.3). The proposed conditions have been successfully used for the quantitation of the analytes in the 0.25–50.0 μg L−1 range. The limits of detection (LODs) are in the 0.02–0.1 μg L−1 range, and the intraday and interday relative standard deviation (RSD) coefficients are ≤9 and ≤17 %, respectively. The reliability of the described method has been tested by analyzing several real water samples. The proposed method can be considered an environmentally friendly and cost-effective alternative for routine monitoring of triazines and their degradation products in waters.

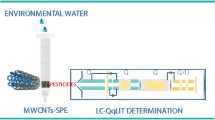
Schematic diagram of the system used for the analysis of triazines





Similar content being viewed by others
References
Directive 2008/105/EC of the European Parliament and Council on environmental quality standards in the field of water policy
Directive 98/83/EC of the European Parliament and Council on the quality of water intended for human consumption
Auersperger P, Lah K, Kus J, Marsel J (2005) High precision procedure for determination of selected herbicides and their degradation products in drinking water by solid-phase extraction and gas chromatography–mass spectrometry. J Chromatogr A 1088:234–241
Ferrer I, Thurman EM (2007) Multi-residue method for the analysis of 101 pesticides and their degradates in food and water samples by liquid chromatography/time-of-flight mass spectrometry. J Chromatogr A 1175:24–37
Nurmi J, Pellinen J (2011) Multiresidue method for the analysis of emerging contaminants in wastewater by ultra performance liquid chromatography–time-of-flight mass spectrometry. J Chromatogr A 1218:6712–6719
Köck-Schulmeyer K, Villagrasa M, López de Alda M, Céspedes-Sánchez R, Ventura F, Barceló D (2013) Occurrence and behavior of pesticides in wastewater treatment plants and their environmental impact. Sci Total Environ 458–460:466–476
Masiá A, Campo J, Vázquez-Roig P, Blanco C, Picó Y (2013) Screening of currently used pesticides in water, sediments and biota of the Guadalquivir River Basin (Spain). J Hazard Mater 263:95–104
Huang SD, Huang HI, Sung YH (2004) Analysis of triazine in water samples by solid-phase microextraction coupled with high-performance liquid chromatography. Talanta 64:887–893
Cháfer-Pericás C, Herráez-Hernández R, Campíns-Falcó P (2006) On-fibre solid-phase microextraction coupled to conventional liquid chromatography versus in-tube solid-phase microextraction coupled to capillary liquid chromatography for the screening analysis of triazines in water samples. J Chromatogr A 1125:159–171
Rocha C, Pappas EA, Huang C (2008) Determination of trace triazine and chloroacetamide herbicides in tile-fed drainage ditch water using solid-phase microextraction coupled with GC–MS. Environ Pollut 152:239–244
Sanchez-Ortega A, Unceta N, Gómez-Caballero A, Sampedro MC, Akesolo U, Goicolea MA, Barrio RJ (2009) Sensitive determination of triazines in underground waters using stir bar sorptive extraction directly coupled to automated thermal desorption and gas chromatography–mass spectrometry. Anal Chim Acta 641:110–116
Nagaraju D, Huang SD (2007) Determination of triazine herbicides in aqueous samples by dispersive liquid–liquid microextraction with gas chromatography–ion trap mass spectrometry. J Chromatogr A 1161:89–97
Sabik H, Jeannot R, Rondeau B (2000) Multiresidue methods using solid-phase extraction techniques for monitoring priority pesticides, including triazines and degradation products, in ground and surface waters. J Chromatogr A 885:217–236
Loos R, Locoro G, Contini S (2010) Occurrence of polar organic contaminants in the dissolved water phase of the Danube River and its major tributaries using SPE-LC-MS2 analysis. Water Res 44:2325–2335
Loos R, Locoro G, Comero S, Contini S, Schwesig D, Werres F, Balsaa P, Gans O, Weiss S, Blaha L, Bolchi M, Gawlik BM (2010) Pan-European survey on the occurrence of selected polar organic persistent pollutants in ground water. Water Res 44:4115–4126
Benvenuto F, Marín JM, Sancho JV, Canobbio S, Mezzanotte V, Hernández F (2010) Simultaneous determination of triazines and their main transformation products in surface and urban wastewater by ultra-high-pressure liquid chromatography–tandem mass spectrometry. Anal Bioanal Chem 397:2791–2805
Campíns-Falcó P, Verdú-Andrés J, Sevillano-Cabeza A, Molins-Legua C, Herráez-Hernández R (2008) New micromethod combining miniaturized matrix solid-phase dispersion and in-tube in-valve solid-phase microextraction for estimating polycyclic aromatic hydrocarbons in bivalves. J Chromatogr A 1211:13–21
Campíns-Falcó P, Verdú-Andrés J, Sevillano-Cabeza A, Herráez-Hernández R, Molins-Legua C, Moliner-Martínez Y (2010) In-tube solid-phase microextraction coupled by in valve mode to capillary LC-DAD: improving detectability to multiresidue organic pollutants analysis in several whole waters. J Chromatogr A 1217:2695–2702
Cháfer-Pericás C, Herráez-Hernández R, Campíns-Falco P (2007) In-tube solid-phase microextraction-capillary liquid chromatography as a solution for the screening analysis of organophosphorus pesticides in untreated environmental water samples. J Chromatogr A 1141:10–21
Moliner-Martínez Y, Molins-Legua C, Verdú-Andrés J, Herráez-Hernández R, Campíns-Falcó P (2011) Advantages of monolithic over particulate columns for multiresidue analysis of organic pollutants by in-tube solid-phase microextraction coupled to capillary liquid chromatography. J Chromatogr A 1218:6256–6262
Fontanals N, Marcé RM, Borrull F (2007) New materials in sorptive extraction techniques for polar compounds. J Chromatogr A 1152:14–31
Wen Y, Chen L, Li J, Liu D, Chen L (2014) Recent advances in solid-phase sorbents for sample preparation prior to chromatographic analysis. Trends Anal Chem 59:26–41
Tian J, Xu J, Zhu F, Lu T, Su C, Ouyang G (2013) Application of nanomaterials in sample preparation. J Chromatogr A1300:2–16
Wen Y, Chen L, Li J, Ma Y, Xu S, Zhang Z, Niu Z, Choo J (2012) Molecularly imprinted matrix solid-phase dispersion coupled to micellar electrokinetic chromatography for simultaneous determination of triazines in soil, fruit, and vegetable samples. Electrophoresis 33:2454–2463
Pyrzynska K (2013) Use of nanomaterials in sample preparation. Trends Anal Chem 43:100–108
Wang X, Li X, Li Z, Zhang Y, Bai Y, Lu H (2014) Online coupling of in-tube solid-phase microextraction with direct analysis in real time mass spectrometry for rapid determination of triazine herbicides in water using carbon-nanotubes-incorporated polymer monolith. Anal Chem 86:4739–4747
Suárez B, Simonet BM, Cárdenas S, Valcárcel M (2007) Determination of non-steroidal anti-inflammatory drugs in urine by combining an immobilized carboxylated carbon nanotubes minicolumn for solid-phase extraction with capillary electrophoresis-mass spectrometry. J Chromatogr A 1159:203–207
Sombra L, Moliner-Martínez Y, Cárdenas S, Valcárcel M (2008) Carboxylic multi-walled carbon nanotubes as immobilized stationary phase in capillary electrochromatography. Electrophoresis 29:3850–3857
Slentz BE, Penner NA, Lugowska E, Regnier F (2001) Nanoliter capillary electrochromatography columns based on collocated monolithic support structures molded in poly(dimethyl siloxane). Electrophoresis 22:3736–3743
Frankhauser-Noti A, Grob K (2007) Blank problems in trace analysis of diethylhexyl and dibutyl phthalate: investigation of the sources, tips and tricks. Anal Chim Acta 582:353–360
Zhao G, Song S, Wang C, Wu Q, Wang Z (2011) Determination of triazine herbicides in environmental water samples by high-performance liquid chromatography using graphene-coated magnetic nanoparticles as adsorbent. Anal Chim Acta 708:155–159
Ma J, Xiao R, Li J, Yu J, Zhang Y, Chen L (2010) Determination of 16 polycyclic aromatic hydrocarbons in environmental water samples by solid-phase extraction using multi-walled carbon nanotubes as adsorbent coupled with gas chromatography-mass spectrometry. J Chromatogr A 1217:5462–5469
Xu S, Chen L, Li J, Qin W, Ma J (2011) Preparation of hollow porous molecularly imprinted polymers and their applications to solid-phase extraction of triazines in soil samples. J Mater Chem 21:12047–12053
Xu S, Lu H, Chen H (2014) Double water compatible molecularly imprinted polymers applied as solid-phase extraction sorbent for selective preconcentration and determination of triazines in complicated water samples. J Chromatogr A 1350:23–29
Bonansea RI, Amé MV, Wunderlin DA (2013) Determination of priority pesticides in water samples combining SPE and SPME coupled to GC–MS. A case study: Suquía River basin (Argentina). Chemosphere 90:1860–1869
Djozan D, Ebrahimi B (2008) Preparation of new solid phase micro extraction fiber on the basis of atrazine-molecular imprinted polymer: application for GC and GC/MS screening of triazine herbicides in water, rice and onion. Anal Chim Acta 616:152–159
Djozan D, Ebrahimi B, Mahkam M, Farajzadeh MA (2010) Evaluation of a new method for chemical coating of aluminum wire with molecularly imprinted polymer layer. Application for the fabrication of triazines selective solid-phase microextraction fiber. Anal Chim Acta 674:40–48
Hu Y, Wang Y, Hu Y, Li G (2009) Liquid–liquid–solid microextraction based on membrane-protected molecularly imprinted polymer fiber for trace analysis of triazines in complex aqueous samples. J Chromatogr A 1216:8304–8311
See HH, Sanagi MM, Ibrahim WAV, Naim AA (2010) Determination of triazine herbicides using membrane-protected carbon nanotubes solid phase membrane tip extraction prior to micro-liquid chromatography. J Chromatogr A 1217:1767–1772
Bagheri H, Ayazi Z, Es’haghi A, Aghakhani A (2012) Reinforced polydiphenylamine nanocomposite for microextraction in packed syringe of various pesticides. J Chromatogr A 1222:13–21
Wu J, Tragas C, Lord H, Pawliszyn J (2002) Analysis of polar pesticides in water and wine samples by automated in-tube solid-phase microextraction coupled with high-performance liquid chromatography–mass spectrometry. J Chromatogr A 976:357–367
Fan Y, Zhang M, Feng YQ (2005) Poly(acrylamide-vinylpyridine-N,N′-methylene bisacrylamide) monolithic capillary for in-tube solid-phase microextraction coupled to high performance liquid chromatography. J Chromatogr A 1099:84–91
Acknowledgments
The authors are grateful to the Spanish Ministerio de Economia y Competitividad (project CTQ 2011-26760) and to the Generalitat Valenciana (project ACOMP/2013/155) for the financial support received.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 464 kb)
Rights and permissions
About this article
Cite this article
Moliner-Martínez, Y., Serra-Mora, P., Verdú-Andrés, J. et al. Analysis of polar triazines and degradation products in waters by in-tube solid-phase microextraction and capillary chromatography: an environmentally friendly method. Anal Bioanal Chem 407, 1485–1497 (2015). https://doi.org/10.1007/s00216-014-8366-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8366-7