Abstract
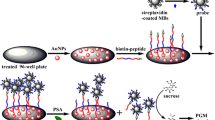
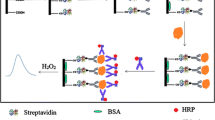
An enzyme-linked immuno-mass spectrometric assay (ELIMSA) with the specific detection probe streptavidin conjugated to alkaline phosphatase catalyzed the production of adenosine from the substrate adenosine monophosphate (AMP) for sensitive quantification of prostate-specific antigen (PSA) by mass spectrometry. Adenosine ionized efficiently and was measured to the femtomole range by dilution and direct analysis with micro-liquid chromatography, electrospray ionization, and mass spectrometry (LC-ESI-MS). The LC-ESI-MS assay for adenosine production was shown to be linear and accurate using internal 13C15N adenosine isotope dilution, internal 13C15N adenosine one-point calibration, and external adenosine standard curves with close agreement. The detection limits of LC-ESI-MS for alkaline phosphatase–streptavidin (AP-SA, ∼190,000 Da) was tested by injecting 0.1 μl of a 1 pg/ml solution, i.e., 100 attograms or 526 yoctomole (5.26E−22) of the alkaline-phosphatase labeled probe on column (about 315 AP-SA molecules). The ELIMSA for PSA was linear and showed strong signals across the picogram per milliliter range and could robustly detect PSA from all of the prostatectomy patients and all of the female plasma samples that ranged as low as 70 pg/ml with strong signals well separated from the background and well within the limit of quantification of the AP-SA probe. The results of the ELIMSA assay for PSA are normal and homogenous when independently replicated with a fresh standard over multiple days, and intra and inter diem assay variation was less than 10 % of the mean. In a blind comparison, ELIMSA showed excellent agreement with, but was more sensitive than, the present gold standard commercial fluorescent ELISA, or ECL-based detection, of PSA from normal and prostatectomy samples, respectively.





Similar content being viewed by others
Abbreviations
- AMP:
-
Adenosine monophosphate
- AP-SA:
-
Alkaline phosphatase–streptavidin
- ECL:
-
Enhanced chemiluminescence
- ELISA:
-
Enzyme-linked immunosorbent assay
- ELIMSA:
-
Enzyme-linked mass spectrometric assay
- LC-ESI-MS:
-
Liquid chromatography electrospray ionization and mass spectrometry
- PSA:
-
Prostate-specific antigen
- SIM:
-
Single ion monitoring
References
Engvall E, Perlman P (1971) Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8:871–874
Van Weemen BK, Schuurs AH (1971) Immunoassay using antigen–enzyme conjugates. FEBS Lett 15:232–236
Sun X, Jin W (2003) Catalysis-electrochemical determination of zeptomole enzyme and its application for single-cell analysis. Anal Chem 75:6050–6055
Cook DB, Self CH (1993) Determination of one thousandth of an attomole (1 zeptomole) of alkaline phosphatase: application in an immunoassay of proinsulin. Clin Chem 39:965–971
Florentinus-Mefailoski A, Safi F, Marshall JG (2014) Enzyme linked immuno mass spectrometric assay (ELIMSA). J Proteomics 96:343–352
Bothner B, Chavez R, Wei J, Strupp C, Phung Q, Schneemann A, Siuzdak G (2000) Monitoring enzyme catalysis with mass spectrometry. J Biol Chem 275:13455–13459
Pris AD, Mondello FJ, Wroczynski RJ, Murray AJ, Boudries H, Surman CM, Paxon TL (2009) Improved specific biodetection with ion trap mobility spectrometry (ITMS): a 10-min, multiplexed, immunomagnetic ELISA. Anal Chem 81:9948–9954
Hempen C, van Leeuwen SM, Luftmann H, Karst U (2005) Liquid chromatographic/mass spectrometric investigation on the reaction products in the peroxidase-catalyzed oxidation of o-phenylenediamine by hydrogen peroxide. Anal Bioanal Chem 382:234–238
Orsin F, Shulman S (1971) The antigens and autoantigens of the seminal vesicle. I. Immunochemical studies on guinea pig vesicular fluid. J Exp Med 134:120–140
Black MH, Grass CL, Leinonen J, Stenman UH, Diamandis EP (1999) Characterization of monoclonal antibodies for prostate-specific antigen and development of highly sensitive free prostate-specific antigen assays. Clin Chem 45:347–354
Kulasingam V, Smith CR, Batruch I, Buckler A, Jeffery DA, Diamandis EP (2008) “Product ion monitoring” assay for prostate-specific antigen in serum using a linear ion-trap. J Proteome Res 7:640–647
Diamandis EP (1988) Immunoassays with time-resolved fluorescence spectroscopy: principles and applications. Clin Biochem 21:139–150
Chikkaveeraiah BV, Bhirde AA, Morgan NY, Eden HS, Chen X (2012) Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 6:6546–6561
Marshall J, B. P., Schmit JC and Betsou F. (2014) Creation of a federated database of blood proteins: a powerful new tool for finding and characterizing biomarkers in serum. Clinical Proteomics 11
Banks JF Jr, Shen S, Whitehouse CM, Fenn JB (1994) Ultrasonically assisted electrospray ionization for LC/MS determination of nucleosides from a transfer RNA digest. Anal Chem 66:406–414
Stephan C, Kramer J, Meyer HA, Kristiansen G, Ziemer S, Deger S, Lein M, Loening SA, Jung K (2007) Different prostate-specific antigen assays give different results on the same blood sample: an obstacle to recommending uniform limits for prostate biopsies. BJU Int 99:1427–1431
Chelius D, Huhmer AF, Shieh CH, Lehmberg E, Traina JA, Slattery TK, Pungor E Jr (2002) Analysis of the adenovirus type 5 proteome by liquid chromatography and tandem mass spectrometry methods. J Proteome Res 1:501–513
Schwartz JC, Senko MW, Syka JE (2002) A two-dimensional quadrupole ion trap mass spectrometer. J Am Soc Mass Spectrom 13:659–669
Florentinus AK, Bowden P, Sardana G, Diamandis EP, Marshall JG (2012) Identification and quantification of peptides and proteins secreted from prostate epithelial cells by unbiased liquid chromatography tandem mass spectrometry using goodness of fit and analysis of variance. J Proteomics 75:1303–1317
Bowden P, Thavarajah T, Zhu P, McDonell M, Thiele H, Marshall JG (2012) Quantitative statistical analysis of standard and human blood proteins from liquid chromatography, electrospray ionization, and tandem mass spectrometry. J Proteome Res 11:2032–2047
Florentinus AK, Jankowski A, Petrenko V, Bowden P, Marshall JG (2011) The Fc receptor–cytoskeleton complex from human neutrophils. J Proteomics 75:450–468
Belov ME, Gorshkov MV, Udseth HR, Anderson GA, Tolmachev AV, Prior DC, Harkewicz R, Smith RD (2000) Initial implementation of an electrodynamic ion funnel with Fourier transform ion cyclotron resonance mass spectrometry. J Am Soc Mass Spectrom 11:19–23
Kandiah M, Urban PL (2013) Advances in ultrasensitive mass spectrometry of organic molecules. Chem Soc Rev 42:5299–5322
Munge B, Liu G, Collins G, Wang J (2005) Multiple enzyme layers on carbon nanotubes for electrochemical detection down to 80 DNA copies. Anal Chem 77:4662–4666
Sun X, Gao N, Jin W (2006) Monitoring yoctomole alkaline phosphatase by capillary electrophoresis with on-capillary catalysis-electrochemical detection. Anal Chim Acta 571:30–33
Zhang H, Li XF, Le XC (2012) Binding-induced DNA assembly and its application to yoctomole detection of proteins. Anal Chem 84:877–884
Mullis KB (1990) Target amplification for DNA analysis by the polymerase chain reaction. Ann Biol Clin (Paris) 48:579–582
Rutledge RG (2004) Sigmoidal curve-fitting redefines quantitative real-time PCR with the prospective of developing automated high-throughput applications. Nucleic Acids Res 32:e178
Melegos DN, Diamandis EP (1998) Is prostate-specific antigen present in female serum? Clin Chem 44:691–692
Aebersold R, Mann M (2003) Mass spectrometry-based proteomics. Nature 422:198–207
Salehpour M, Possnert G, Bryhni H (2008) Subattomole sensitivity in biological accelerator mass spectrometry. Anal Chem 80:3515–3521
Liu G, Wang J, Kim J, Jan MR, Collins GE (2004) Electrochemical coding for multiplexed immunoassays of proteins. Anal Chem 76:7126–7130
Lohmann, W., Hayen, H., and Karst, U. (2008) Covalent protein modification by reactive drug metabolites using online electrochemistry/liquid chromatography/mass spectrometry. Anal Chem
Rozet E, Morello R, Lecomte F, Martin GB, Chiap P, Crommen J, Boos KS, Hubert P (2006) Performances of a multidimensional on-line SPE-LC-ECD method for the determination of three major catecholamines in native human urine: validation, risk and uncertainty assessments. J Chromatogr B: Analyt Technol Biomed Life Sci 844:251–260
Takatsy A, Boddi K, Nagy L, Nagy G, Szabo S, Marko L, Wittmann I, Ohmacht R, Ringer T, Bonn GK, Gjerde D, Szabo Z (2009) Enrichment of Amadori products derived from the nonenzymatic glycation of proteins using microscale boronate affinity chromatography. Anal Biochem 393:8–22
Tang CK, Vaze A, Rusling JF (2012) Fabrication of immunosensor microwell arrays from gold compact discs for detection of cancer biomarker proteins. Lab Chip 12:281–286
Valentini F, Compagnone D, Gentili A, Palleschi G (2002) An electrochemical ELISA procedure for the screening of 17beta-estradiol in urban waste waters. Analyst 127:1333–1337
Zhang S, Yang J, Lin J (2008) 3,3′-diaminobenzidine (DAB)-H2O2-HRP voltammetric enzyme-linked immunoassay for the detection of carcionembryonic antigen. Bioelectrochemistry 72:47–52
Shi T, Sun X, Gao Y, Fillmore TL, Schepmoes AA, Zhao R, He J, Moore RJ, Kagan J, Rodland KD, Liu T, Liu AY, Smith RD, Tang K, Camp DG 2nd, Qian WJ (2013) Targeted quantification of low ng/mL level proteins in human serum without immunoaffinity depletion. J Proteome Res 12:3353–3361
Kricka LJ (1993) Ultrasensitive immunoassay techniques. Clin Biochem 26:325–331
Tucholska M, Bowden P, Jacks K, Zhu P, Furesz S, Dumbrovsky M, Marshall J (2009) Human serum proteins fractionated by preparative partition chromatography prior to LC-ESI-MS/MS. J Proteome Res 8:1143–1155
Tucholska M, Florentinus A, Williams D, Marshall JG (2010) The endogenous peptides of normal human serum extracted from the acetonitrile-insoluble precipitate using modified aqueous buffer with analysis by LC-ESI-Paul ion trap and Qq-TOF. J Proteomics 73:1254–1269
Marshall J, Jankowski A, Furesz S, Kireeva I, Barker L, Dombrovsky M, Zhu W, Jacks K, Ingratta L, Bruin J, Kristensen E, Zhang R, Stanton E, Takahashi M, Jackowski G (2004) Human serum proteins preseparated by electrophoresis or chromatography followed by tandem mass spectrometry. J Proteome Res 3:364–382
Addona TA, Abbatiello SE, Schilling B, Skates SJ, Mani DR, Bunk DM, Spiegelman CH, Zimmerman LJ, Ham AJ, Keshishian H, Hall SC, Allen S, Blackman RK, Borchers CH, Buck C, Cardasis HL, Cusack MP, Dodder NG, Gibson BW, Held JM, Hiltke T, Jackson A, Johansen EB, Kinsinger CR, Li J, Mesri M, Neubert TA, Niles RK, Pulsipher TC, Ransohoff D, Rodriguez H, Rudnick PA, Smith D, Tabb DL, Tegeler TJ, Variyath AM, Vega-Montoto LJ, Wahlander A, Waldemarson S, Wang M, Whiteaker JR, Zhao L, Anderson NL, Fisher SJ, Liebler DC, Paulovich AG, Regnier FE, Tempst P, Carr SA (2009) Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotechnol 27:633–641
Acknowledgment
This work was supported by a Discovery Grant from the Natural Science and Engineering Research Council of Canada to JGM.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 704 kb)
Rights and permissions
About this article
Cite this article
Florentinus-Mefailoski, A., Soosaipillai, A., Dufresne, J. et al. An enzyme-linked immuno-mass spectrometric assay with the substrate adenosine monophosphate. Anal Bioanal Chem 407, 1119–1130 (2015). https://doi.org/10.1007/s00216-014-8323-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8323-5