Abstract
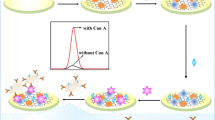
A highly sensitive electrochemiluminescent (ECL) biosensor was designed for the detection of concanavalin A (ConA) based on glucose oxidase (GOx) as a recognition element by carbohydrate–lectin biospecific interaction, and poly(ethylenimine) (PEI) reduced graphene and hollow gold nanoparticles (HAuNPs) as supporting matrix and signal amplifier. The modification process and detection principle of the biosensor are briefly described as follows. First, PEI reduced graphene oxide with abundant amino groups was cast onto the surface of glassy carbon electrode to adsorb HAuNPs for improving the signal intensity in luminol/H2O2 ECL system. Next, GOx was further assembled onto the electrode by the interaction between Au and –NH2. In the presence of glucose in the detection solution, GOx catalyzed glucose to generate H2O2 in situ, which served as a co-reactant of luminol to enhance ECL signal of luminol. Based on the fact that ConA could result in a decrease in ECL signal when immobilized on the electrode, an ECL biosensor was prepared for the determination of ConA. The ECL signal intensity was linear with the logarithm of ConA concentration and the linear range was from 1.0 to 20 ng/mL with a low detection limit of 0.31 ng/mL (signal to noise ratio =3). This strategy led to a nearly 1000-fold improvement in detection limit for ConA assays compared with previously reported method, thus exhibiting a great potential application in sensitive bioassays of ConA.





Similar content being viewed by others
References
Loaiza OA, Lamas-Ardisana PJ, Jubete E, Ochoteco E, Loinaz I, Cabañero G, García I, Penades S (2011) Nanostructured disposable impedimetric sensors as tools for specific biomolecular interactions: sensitive recognition of concanavalin A. Anal Chem 83:2987–2995
Bellapadrona G, Tesler AB, Grünstein D, Hossain LH, Kikkeri R, Seeberger PH, Vaskevich A, Rubinstein I (2012) Optimization of localized surface plasmon resonance transducers for studying carbohydrate-protein interactions. Anal Chem 84:232–240
Yonzon CR, Jeoung E, Zou S, Schatz GC, Mrksich M, Duyne RPV (2004) A comparative analysis of localized and propagating surface plasmon resonance sensors: the binding of concanavalin A to a monosaccharide functionalized self-assembled monolayer. J Am Chem Soc 126:12669–12676
Li WJ, Yuan R, Chai YQ (2010) Determination of glucose using pseudobienzyme channeling based on sugar–lectin biospecific interactions in a novel organic-inorganic composite matrix. J Phys Chem C 114:21397–21404
Zou L, Pang HL, Chan PH, Huang ZS, Gu LQ, Wong KY (2008) Trityl-derivatized carbohydrates immobilized on a polystyrene microplate. Carbohydr Res 343:2932–2938
Huang CC, Chen CT, Shiang YC, Lin ZH, Chang HT (2009) Synthesis of fluorescent carbohydrate-protected Au nanodots for detection of concanavalin A and escherichia coli. Anal Chem 81:875–882
Guo CX, Boullanger P, Jiang L, Liu T (2007) Highly sensitive gold nanoparticles biosensor chips modified with a self-assembled bilayer for detection of Con A. Biosens Bioelectron 22:1830–1834
Hone DC, Haines AH, Russell DA (2003) rapid, quantitative colorimetric detection of a lectin using mannose-stabilized gold nanoparticles. Langmuir 19:7141–7144
Huang CF, Yao GH, Liang RP, Qiu JD (2013) Graphene oxide and dextran capped gold nanoparticles based surface plasmon resonance sensor for sensitive detection of concanavalin A. Biosens Bioelectron 50:305–310
Hu F, Chen S, Wang C, Yuan R, Xiang Y, Wang C (2012) Multi-wall carbon nanotube-polyaniline biosensor based on lectin–carbohydrate affinity for ultrasensitive detection of Con A. Biosens Bioelectron 34:202–207
Guo C, Boullanger P, Jiang L, Liu T (2008) One-step immobilization of alkanethiol/glycolipid vesicles onto gold electrode: amperometric detection of Concanavalin A. Colloids Surf, B 62:146–150
Xu S, Liu Y, Wang T, Li J (2011) Positive potential operation of a cathodic electrogenerated chemiluminescence immunosensor based on luminol and graphene for cancer biomarker detection. Anal Chem 83:3817–3823
Wang HJ, Yuan R, Chai YQ, Niu H, Cao YL, Liu H (2012) Bi-enzyme synergetic catalysis to in situ generate coreactant of peroxydisulfate solution for ultrasensitive electrochemiluminescence immunoassay. Biosens Bioelectron 37:6–10
Cao YL, Yuan R, Chai YQ, Mao L, Niu H, Liu HJ, Zhuo Y (2012) Ultrasensitive luminol electrochemiluminescence for protein detection based on in situ generated hydrogen peroxide as coreactant with glucose oxidase anchored AuNPs@MWCNTs labeling. Biosens Bioelectron 31:305–309
Jiang XY, Chai YQ, Wang H, Yuan R (2014) Electrochemiluminescence of luminol enhanced by the synergetic catalysis of hemin and silver nanoparticles for sensitive protein detection. Biosens Bioelectron 54:20–29
Chen D, Feng H, Li J (2012) Graphene oxide: preparation, functionalization, and electrochemical applications. Chem Rev 112:6027–6053
Valentini F, Carbone M, Palleschi G (2013) Graphene oxide nanoribbons (GNO), reduced graphene nanoribbons (GNR), and multi-layers of oxidized graphene functionalized with ionic liquids (GO–IL) for assembly of miniaturized electrochemical devices. Anal Bioanal Chem 405:3449–3474
Zhang Y, Dai WJ, Liu F, Li L, Li M, Ge SG, Yan M, Yu JH (2013) Ultrasensitive electrochemiluminescent immunosensor based on dual signal amplification strategy of gold nanoparticles-dotted graphene composites and CdTe quantum dots coated silica nanoparticles. Anal Bioanal Chem 405:4921–4929
Wang HB, Ou LJ, Huang KJ, Wen XG, Wang LL, Liu YM (2013) A sensitive biosensing strategy for DNA detection based on graphene oxide and T7 exonuclease assisted target recycling amplification. J Chem 91:1266–1271
Szunerits S, Maalouli N, Wijaya E, Vilcot JP, Boukherroub R (2013) Recent advances in the development of graphene-based surface plasmon resonance (SPR) interfaces. Anal Bioanal Chem 405:1435–1443
Xu SJ, Liu Y, Wang TH, Li JH (2010) Highly sensitive electrogenerated chemiluminescence biosensor in profiling protein kinase activity and inhibition using gold nanoparticle as signal transduction probes. Anal Chem 82:9566–9572
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241:20–22
Schwartzberg AM, Oshiro TY, Zhang JZ, Huser T, Talley CE (2006) Improving nanoprobes using surface-enhanced raman scattering from 30-nm hollow gold particles. Anal Chem 78:4732–4736
Cao LY, Liu YL, Zhang BH, Lu LH (2010) In situ controllable growth of prussian blue nanocubes on reduced graphene oxide: facile synthesis and their application as enhanced annoelectrocatalyst for H2O2 reduction. ACS Appl Mat Interfaces 2:2339–2346
Li LL, Liu KP, Yang GH, Wang CM, Zhang JR, Zhu JJ (2011) Fabrication of graphene–quantum dots composites for sensitive electrogenerated chemiluminescence Immunosensing. Adv Funct Mater 21:869–878
Lee DW, De Los Santos LV, Seo JW, Leon Felix L, Bustamante DA, Cole JM, Barnes CHW (2010) The structure of graphite oxide: investigation of its surface chemical groups. J Phys Chem B 114:5723–5728
Jiang L, Gao L (2003) Modified carbon nanotubes: an effective way to selective attachment of gold nanoparticles. Carbon 41:2923–2929
Acknowledgments
This work was financially supported by the NNSF of China (21075100, 21275119, 21105081), Ministry of Education of China (Project 708073), Research Fund for the Doctoral Program of Higher Education (RFDP) (20110182120010), Natural Science Foundation of Chongqing City (CSTC-2011BA7003, CSTC-2010BB4121), State Key Laboratory of Silkworm Genome Biology (sklsgb2013012), and the Fundamental Research Funds for the Central Universities (XDJK2013A008, XDJK2013A27), China.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 57.8 kb)
Rights and permissions
About this article
Cite this article
Zhang, J., Chen, S., Ruo, Y. et al. An ultrasensitive electrochemiluminescent biosensor for the detection of concanavalin A based on poly(ethylenimine) reduced graphene oxide and hollow gold nanoparticles. Anal Bioanal Chem 407, 447–453 (2015). https://doi.org/10.1007/s00216-014-8290-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8290-x