Abstract
Ecgonine is suggested to be a promising marker of cocaine (COC) ingestion. A combined mass spectrometry (MS) and tandem MS (MS/MS) method was developed to simultaneously determine ecgonine and seven other metabolites of cocaine in human urine and whole blood with ultra-high-pressure liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. The compounds were extracted from as little as 100 μL of sample by solid-phase extraction with a 96-well μElution solid-phase extraction plate. The protonated molecules or fragment ions at accurate mass acquired in MS mode were used to quantify specific analytes, following by dedicated MS/MS identification. The assay was linear in the range from 5 to 50-100 ng/mL for urine samples, except for ecgonine methyl ester (10-200 ng/mL) and ecgonine (40-400 ng/mL), and was linear from 1-2 to 50 ng/mL for whole blood samples, except for ecgonine methyl ester (20-1,000 ng/mL) and ecgonine (40-2,000 ng/mL). The correlation coefficients were all greater than 0.99. The limits of detection ranged from 0.2 to 16 ng/mL, and the lower limits of quantification ranged from 1 to 40 ng/mL. The repeatability and intermediate precision were 18.1 % or less. The accuracy was in the range from 80.0 to 122.9 %, process efficiencies were in the range from 8.6 to 177.4 %, matrix effects were in the range from 28.7 to 171.0 %, and extraction recoveries were in the range from 41.0 to 114.3 %, except for ecgonine (12.8 % and 9.3 % at low and high concentrations, respectively). This method was highly sensitive in comparison with previously published methods. The validated method was successfully applied to the analysis of real samples derived from forensic cases, and the results verified that, on the basis of data from four positive samples, ecgonine is a promising marker of cocaine ingestion.

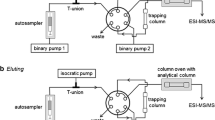
Procedure for the determination of ecgonine and seven other cocaine metabolites in human urine and whole blood using a combined mass spectrometry and tandem MS method aIer the solid‐phase extraction of the anaytes



Similar content being viewed by others
References
United Nations Office on Drugs and Crime (2012) World drug report 2012. http://www.unodc.org/unodc/en/data-and-analysis/WDR-2012.html. Accessed May 2013
Duer WC, Spitz DJ, McFarland S (2006) Relationships between concentrations of cocaine and its hydrolysates in peripheral blood, heart blood, vitreous humor and urine. J Forensic Sci 51(2):421–425
Cardona PS, Chaturvedi AK, Soper JW, Canfield DV (2006) Simultaneous analyses of cocaine, cocaethylene, and their possible metabolic and pyrolytic products. Forensic Sci Int 157(1):46–56
Paul BD, Lalani S, Bosy T, Jacobs AJ, Huestis MA (2005) Concentration profiles of cocaine, pyrolytic methyl ecgonidine and thirteen metabolites in human blood and urine: determination by gas chromatography-mass spectrometry. Biomed Chromatogr 19(9):677–688. doi:10.1002/bmc.495
Smith ML, Shimomura E, Paul BD, Cone EJ, Darwin WD, Huestis MA (2010) Urinary excretion of ecgonine and five other cocaine metabolites following controlled oral, intravenous, intranasal, and smoked administration of cocaine. J Anal Toxicol 34(2):57–63
Xia Y, Wang P, Bartlett MG, Solomon HM, Busch KL (2000) An LC-MS-MS method for the comprehensive analysis of cocaine and cocaine metabolites in meconium. Anal Chem 72(4):764–771
Skopp G, Klingmann A, Potsch L, Mattern R (2001) In vitro stability of cocaine in whole blood and plasma including ecgonine as a target analyte. Ther Drug Monit 23(2):174–181
Nishikawa M, Nakajima K, Tatsuno M, Kasuya F, Igarashi K, Fukui M, Tsuchihashi H (1994) The analysis of cocaine and its metabolites by liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry (LC/APCI-MS). Forensic Sci Int 66(3):149–158
Klingmann A, Skopp G, Aderjan R (2001) Analysis of cocaine, benzoylecgonine, ecogonine methyl ester, and ecgonine by high-pressure liquid chromatography-API mass spectrometry and application to a short-term degradation study of cocaine in plasma. J Anal Toxicol 25(6):425–430
Jagerdeo E, Montgomery MA, Lebeau MA, Sibum M (2008) An automated SPE/LC/MS/MS method for the analysis of cocaine and metabolites in whole blood. J Chromatogr B Anal Technol Biomed Life Sci 874(1–2):15–20
Peterson KL, Logan BK, Christian GD (1995) Detection of cocaine and its polar transformation products and metabolites in human urine. Forensic Sci Int 73(3):183–196
Hornbeck CL, Barton KM, Czarny RJ (1995) Urine concentrations of ecgonine from specimens with low benzoylecgonine levels using a new ecgonine assay. J Anal Toxicol 19(3):133–138
Jagerdeo E, Montgomery MA, Sibum M, Sasaki TA, LeBeau MA (2008) Rapid analysis of cocaine and metabolites in urine using a completely automated solid-phase extraction-high-performance liquid chromatography-tandem mass spectrometry method. J Anal Toxicol 32(8):570–576
Mallet CR, Lu Z, Fisk R, Mazzeo JR, Neue UD (2003) Performance of an ultra-low elution-volume 96-well plate: drug discovery and development applications. Rapid Commun Mass Spectrom 17(2):163–170
Badoud F, Grata E, Boccard J, Guillarme D, Veuthey JL, Rudaz S, Saugy M (2011) Quantification of glucuronidated and sulfated steroids in human urine by ultra-high pressure liquid chromatography quadrupole time-of-flight mass spectrometry. Anal Bioanal Chem 400(2):503–516
Badoud F, Grata E, Perrenoud L, Saugy M, Rudaz S, Veuthey JL (2010) Fast analysis of doping agents in urine by ultra-high-pressure liquid chromatography-quadrupole time-of-flight mass spectrometry. II: confirmatory analysis. J Chromatogr A 18(25):4109–4119
Commission of the European Communities (2002) 2002/657/EC: Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (text with EEA relevance) (notified under document number C(2002) 3044). http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32002D0657:EN:HTML. Accessed May 2013
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75(13):3019–3030
Society of Forensic Toxicologists and the Toxicology Section of the American Academy of Forensic Sciences (2006) Forensic toxicology laboratory guidelines 2006 version. http://www.soft-tox.org/files/Guidelines_2006_Final.pdf. Accessed May 2013
Bijlsma L, Sancho JV, Hernandez F, Niessen WM (2011) Fragmentation pathways of drugs of abuse and their metabolites based on QTOF MS/MS and MS(E) accurate-mass spectra. J Mass Spectrom 46(9):865–875
Wang P, Bartlett MG (1998) Collision–induced dissociation mass spectra of cocaine, and its metabolites and pyrolysis products. J Mass Spectrom 33(10):961–967
Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, Shah VP, Skelly JP, Swann PG, Weiner R (2007) Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm Res 24(10):1962–1973
Marchi I, Viette V, Badoud F, Fathi M, Saugy M, Rudaz S, Veuthey JL (2010) Characterization and classification of matrix effects in biological samples analyses. J Chromatogr A 18(25):4071–4078
Van Loco J, Elskens M, Croux C, Beernaert H (2002) Linearity of calibration curves: use and misuse of the correlation coefficient. Accred Qual Assur 7(7):281–285
Hibbert DB (2007) Quality assurance for the analytical chemistry laboratory. Oxford University Press, Oxford
Food and Drug Administration (2001) Guidance for industry: bioanalytical method validation. US Department of Health and Human Services, Food and Drug Administration, Centre for Drug Evaluation and Research, Rockville
Logan BK, Peterson KL (1994) The origin and significance of ecgonine methyl ester in blood samples. J Anal Toxicol 18(2):124–125
Isenschmid DS, Levine BS, Caplan YH (1989) A comprehensive study of the stability of cocaine and its metabolites. J Anal Toxicol 13(5):250–256
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xiong, L., Wang, R., Liang, C. et al. Determination of ecgonine and seven other cocaine metabolites in human urine and whole blood by ultra-high-pressure liquid chromatography–quadrupole time-of-flight mass spectrometry. Anal Bioanal Chem 405, 9805–9816 (2013). https://doi.org/10.1007/s00216-013-7417-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7417-9