Abstract
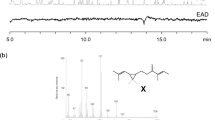
trans-11,12-Epoxy-(6Z,9Z)-6,9-henicosadiene (posticlure) has been identified from a pheromone gland of the lymantriid species, Orgyia postica. Since the diversity of Lepidoptera suggests that some species utilize the structure-related epoxy compound as a sex pheromone component, epoxydienes and epoxytrienes derived from (6Z,9Z,11E)-6,9,11-trienes and (3Z,6Z,9Z,11E)-3,6,9,11-tetraenes with a C19–C21 chain were systematically synthesized and the chemical data were accumulated in order to contribute to a new pheromone research. Peracid oxidation of each triene and each tetraene produced, respectively, a mixture of three epoxydienes (cis-6,7-epoxy-9,11-diene; cis-9,10-epoxy-6,11-diene; and trans-11,12-epoxy-6,9-diene) and four epoxytrienes (cis-3,4-epoxy-6,9,11-triene; cis-6,7-epoxy-3,9,11-triene; cis-9,10-epoxy-3,6,11-triene; and trans-11,12-epoxy-3,6,9-triene). While the 9,10-epoxy compounds were unstable and, interestingly, converted into 9-ketone derivatives after chromatography over SiO2, each positional isomer was isolated by HPLC equipped with an ODS column, and the chemical structure was determined by NMR analysis. On the GC-MS analysis with a DB-23 column, the positional isomers were also eluted separately and characteristic mass spectra were proposed. By comparing the spectral data of the epoxy compounds with a different carbon chain, diagnostic fragment ions reflecting the chemical structure were determined as follows: m/z 79, 109, 113, and M-114 for the 6,7-epoxydienes; m/z 69, 97, 111, 139, and M-111 for the 9,10-epoxydienes; m/z 57, 79, 109, 136, M-151, and M-111 for the 11,12-epoxydienes; m/z 79, 91, 105, and 119 for the 3,4-epoxytrienes; m/z 79, 124, M-124, M-96, and M-69 for the 6,7-epoxytrienes; m/z 79, 95, 109, 137, and M-108 for the 9,10-epoxytrienes; and m/z 79, 134, M-149, M-109, and M-95 for the 11,12-epoxytrienes.




Similar content being viewed by others
References
Ando T (2013) Internet database: http://www.tuat.ac.jp/~antetsu/LepiPheroList.htm
El-Sayed AM (2013) Internet database: http://www.pherobase.com/
Ando T, Inomata S, Yamamoto M (2004) Lepidopteran sex pheromones. Topics Current Chem 239:51–96
Jurenka R (2004) Insect pheromone biosynthesis. Topics Current Chem 239:97–132
Millar JG (2000) Polyene hydrocarbons and epoxides: a second major class of lepidopteran sex attractant pheromones. Annu Rev Entomol 45:575–604
Ando T, Kawai T, Matsuoka K (2008) Epoxyalkenyl sex pheromones produced by female moths in highly evolved groups: biosynthesis and its endocrine regulation. J Pestic Sci 33:17–20
Matsuoka K, Yamamoto M, Yamakawa R, Muramatsu M, Naka H, Kondo Y, Ando T (2008) Identification of novel C20 and C22 trienoic acids from arctiid and geometrid female moths that produce polyenyl type II sex pheromone. J Chem Ecol 34:1437–1445
Roelofs WL, Hill AS, Linn CE, Meinwald J, Jain SC, Herbert HJ, Smith RF (1982) Sex pheromone of the winter moth, a geometrid with unusually low temperature precopulatory responses. Science 217:657–659
Wong JW, Palaniswamy P, Underhill EW, Steck WF, Chisholm MD (1984) Novel sex pheromone components from the fall cankerworm moth, Alsophila pometaria. J Chem Ecol 10:463–473
Yamakawa R, Do ND, Adachi Y, Kinjo M, Ando T (2009) (6Z,9Z,12Z)-6,9,12-Octadecatriene and (3Z,6Z,9Z,12Z)-3,6,9,12-icosatetraene, the novel sex pheromones produced by emerald moths. Tetrahedron Lett 50:4738–4740
Yamamoto M, Yamakawa R, Oga T, Takei Y, Kinjo M, Ando T (2008) Synthesis and chemical characterization of hydrocarbons with a 6,9,11-, 3,6,9,11-, or 1,3,6,9-polyene system, pheromone candidates in Lepidoptera. J Chem Ecol 34:1057–1064
Wakamura S, Arakaki N, Yamamoto M, Hiradate S, Yasui H, Yasuda T, Ando T (2001) Posticlure: a novel trans-epoxide as a sex pheromone component of the tussock moth, Orgyia postica (Walker). Tetrahedron Lett 42:687–689
Wakamura S, Arakaki N, Yamamoto M, Hiradate S, Yasui H, Kinjo K, Yasuda T, Yamazawa H, Ando T (2005) Sex pheromone and related compounds in the Ishigaki and Okinawa strains of the tussock moth Orgyia postica (Walker) (Lepidoptera: Lymantriidae). Biosci Biotechnol Biochem 69:957–965
Ando T, Yamakawa R (2011) Analyses of lepidopteran sex pheromones by mass spectrometry. Trends Anal Chem 30:990–1002
Ando T, Kishi H, Akashio N, Qin X-R, Saito N, Abe H, Hashimoto S (1995) Sex attractants of geometrid and noctuid moths: chemical characterization and field test of monoepoxides of 6,9-dienes and related compounds. J Chem Ecol 21:299–311
Ando T, Ohsawa H, Ueno T, Kishi H, Okamura Y, Hashimoto S (1993) Hydrocarbons with a homoconjugated polyene system and their monoepoxy derivatives: sex attractants of geometrid and noctuid moths distributed in Japan. J Chem Ecol 19:787–798
Yamazawa H, Nakajima N, Wakamura S, Arakaki N, Yamamoto M, Ando T (2001) Synthesis and characterization of diepoxyalkenes derived from (3Z,6Z,9Z)-trienes: lymantriid sex pheromones and their candidates. J Chem Ecol 27:2153–2167
Yamakawa R, Takubo Y, Shibasaki H, Murakami Y, Yamamoto M, Ando T (2012) Characterization of epoxytrienes derived from 3Z,6Z,9Z)-1,3,6,9-tetraenes, sex pheromone components of arctiid moths and related compounds. J Chem Ecol 38:1042–1049
Grant GG, Slessor KN, Liu W, Abou-Zaid MM (2003) (Z,Z)-6,9-heneicosadien-11-one, labile sex pheromone of the whitemarked tussock moth, Orgyia leucostigma. J Chem Ecol 29:589–601
El-Sayed AM, Gibb AR, Suckling DM, Bunn B, Fielder S, Comeskey D, Manning LA, Foster SP, Morris BD, Ando T, Mori K (2005) Identification of sex pheromone components of the painted apple moth: a tussock moth with a thermally labile pheromone component. J Chem Ecol 31:621–646
Acknowledgments
The authors are grateful to Drs. F. Mochizuki and T. Fukumoto of Shin-etsu Chemical Co., Ltd. for supplying 3-hexyn-1,6-diol.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 5681 kb)
Rights and permissions
About this article
Cite this article
Yamamoto, M., Maruyama, R., Murakami, Y. et al. Characterization of posticlure and the structure-related sex pheromone candidates prepared by epoxidation of (6Z,9Z,11E)-6,9,11-trienes and (3Z,6Z,9Z,11E)-3,6,9,11-tetraenes. Anal Bioanal Chem 405, 7405–7414 (2013). https://doi.org/10.1007/s00216-013-7144-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7144-2