Abstract
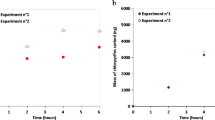
The Chemcatcher passive sampler, which uses Empore™ disks as sampling phase, is frequently used to monitor polar organic chemicals in river water and effluents. Uptake kinetics need to be quantified to calculate time-weighted average concentrations from Chemcatcher field deployments. Information on release kinetics is needed if performance reference compounds (PRCs) are used to quantify the influence of environmental conditions on the uptake. In a series of uptake and elimination experiments, we used Empore™ SDB disks (poly(styrenedivinylbenzene) copolymer modified with sulfonic acid groups) as a sampling phase and 22 compounds with a logK ow (octanol–water partitioning coefficient) range from −2.6 to 3.8. Uptake experiments were conducted in river water or tap water and lasted up to 25 days. Only 1 of 22 compounds (sulfamethoxazole) approached equilibrium in the uptake trials. Other compounds showed continuing non-linear uptake, even after 25 days. All compounds could be released from SDB disks, and desorption was proportionally higher in disks loaded for shorter periods. Desorption showed two-phase characteristics, and desorption was proportionally higher for passively sorbed compounds compared to actively loaded compounds (active loading was performed by pulling spiked river water over SDB disks using vacuum). We hypothesise that the two-phase kinetics and better retention of actively loaded compounds—and compounds loaded for a longer period—may be caused by slow diffusion of chemicals within the polymer. As sorption and desorption did not show isotropic kinetics, it is not possible to develop robust PRCs for adsorbent material like SDB disks.






Similar content being viewed by others
References
Greenwood R, Mills GA, Vrana B (2007) Passive sampling techniques in environmental monitoring. Elsevier, Amsterdam
Huckins JN, Petty JD, Booij K (2006) Monitors of organic chemicals in the environment: semipermeable membrane devices. Springer, New York
Daughton CG (2002) Environmental stewardship and drugs as pollutants. Lancet 360:1035–1036
Sumpter JP (2005) Endocrine disrupters in the aquatic environment: an overview. Acta Hydrochim Hydrobiol 33:9–16
European Commission (2008) Directive 2008/105/EC of the European Parliament and the Council of the European Union (2008) Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC Official Journal of the European Union (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:348:0084:0097:EN:PDF)
European Commission (2012) Proposal for a Directive of the European Parliament and the Council amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. vol http://ec.europa.eu/environment/water/water-dangersub/pdf/com_2011_876.pdf
Zabiegała B, Kot-Wasik A, Urbanowicz M, Namieśnik J (2010) Passive sampling as a tool for obtaining reliable analytical information in environmental quality monitoring. Anal Bioanal Chem 396:273–296
Alvarez DA, Petty JD, Huckins JN, Jones-Lepp TL, Getting DT, Goddard JP, Manahan SE (2004) Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environ Toxicol Chem 23:1640–1648
Kingston JK, Greenwood R, Mills GA, Morrison GM, Persson LB (2000) Development of a novel passive sampling system for the time-averaged measurement of a range of organic pollutants in aquatic environments. J Environ Monit 2:487–495
Escher BI, Quayle P, Muller R, Schreiber U, Mueller JF (2006) Passive sampling of herbicides combined with effect analysis in algae using a novel high-throughput phytotoxicity assay (Maxi-Imaging-PAM). J Environ Monit 8:456–464
MacLeod SL, McClure EL, Wong CS (2007) Laboratory calibration and field deployment of the polar organic chemical integrative sampler for pharmaceuticals and personal care products in wastewater and surface water. Environ Toxicol Chem 26:2517–2529
Vermeirssen ELM, Dietschweiler C, Escher BI, van der Voet J, Hollender J (2012) Transfer kinetics of polar organic compounds over polyethersulfone membranes in the passive samplers POCIS and Chemcatcher. Environ Sci Technol 46:6759–6766
Vrana B, Vermeirssen ELM, Allan IJ, Kohoutek J, Kennedy K, Mills GA, Greenwood R (2010) Passive sampling of emerging pollutants in the aquatic environment: state of the art and perspectives—a position paper of the expert group of the NORMAN network of reference laboratories for monitoring of emerging environmental pollutants
Vermeirssen ELM, Bramaz N, Hollender J, Singer H, Escher BI (2009) Passive sampling combined with ecotoxicological and chemical analysis of pharmaceuticals and biocides—evaluation of three ChemcatcherTM configurations. Water Res 43:903–914
Shaw M, Mueller JF (2009) Time integrative passive sampling: how well do Chemcatchers integrate fluctuating pollutant concentrations? Environ Sci Technol 43:1443–1448
Mazzella N, Lissalde S, Moreira S, Fo D, Mazellier P, Huckins JN (2010) Evaluation of the use of performance reference compounds in an Oasis-HLB adsorbent based passive sampler for improving water concentration estimates of polar herbicides in freshwater. Environ Sci Technol 44:1713–1719
Booij K, Smedes F (2010) An improved method for estimating in situ sampling rates of nonpolar passive samplers. Environ Sci Technol 44:6789–6794
Tran ATK, Hyne RV, Doble P (2007) Calibration of a passive sampling device for time-integrated sampling of hydrophilic herbicides in aquatic environments. Environ Toxicol Chem 26:435–443
Shaw M, Eaglesham G, Mueller JF (2009) Uptake and release of polar compounds in SDB-RPS Empore(TM) disks; implications for their use as passive samplers. Chemosphere 75:1–7
Camilleri J, Morin N, Miège C, Coquery M, Cren-Olivé C (2012) Determination of the uptake and release rates of multifamilies of endocrine disruptor compounds on the polar C18 Chemcatcher. Three potential performance reference compounds to monitor polar pollutants in surface water by integrative sampling. J Chromatogr 1237:37–45
Di Toro DM, Horzempa LM (1982) Reversible and resistant components of PCB adsorption–desorption: isotherms. Environ Sci Technol 16:594–602
Vermeirssen ELM, Asmin J, Escher BI, Kwon J-H, Steimen I, Hollender J (2008) The role of hydrodynamics, matrix and sampling duration in passive sampling of polar compounds with Empore™ SDB-RPS disks. J Environ Monit 10:119–128
Lobpreis T, Vrana B, Dominiak E, Dercová K, Mills GA, Greenwood R (2008) Effect of housing geometry on the performance of Chemcatcher(TM) passive sampler for the monitoring of hydrophobic organic pollutants in water. Environ Pollut 153:706–710
Escher BI, Bramaz N, Quayle P, Rutishauser S, Vermeirssen ELM (2008) Monitoring of the ecotoxicological hazard potential by polar organic micropollutants in sewage treatment plants and surface waters using a mode-of-action based test battery. J Environ Monit 10:622–631
Singer H, Jaus S, Hanke I, Lück A, Hollender J, Alder AC (2010) Determination of biocides and pesticides by on-line solid phase extraction coupled with mass spectrometry and their behaviour in wastewater and surface water. Environ Pollut 158:3054–3064
Vrana B, Mills GA, Dominiak E, Greenwood R (2006) Calibration of the Chemcatcher passive sampler for the monitoring of priority organic pollutants in water. Environ Pollut 142:333–343
Stephens BS, Kapernick A, Eaglesham G, Mueller J (2005) Aquatic passive sampling of herbicides on naked particle loaded membranes: accelerated measurement and empirical estimation of kinetic parameters. Environ Sci Technol 39:8891–8897
Finšgar M, Milošev I (2010) Inhibition of copper corrosion by 1,2,3-benzotriazole: a review. Corros Sci 52:2737–2749
Schmitt P, Poiger T, Simon R, Freitag D, Kettrup A, Garrison AW (1997) Simultaneous determination of ionization constants and isoelectric points of 12 hydroxy-s-triazines by capillary zone electrophoresis and capillary isoelectric focusing. Anal Chem 69:2559–2566
Hansch C, Leo A, Hoekman D (1995) Exploring QSAR. Hydrophobic, Electronic and Steric Constants. ACS Professional Reference Book. American Chemical Society, Washington
Rusina TP, Smedes F, Klanova J, Booij K, Holoubek I (2007) Polymer selection for passive sampling: a comparison of critical properties. Chemosphere 68:1344–1351
Rusina TP, Smedes F, Koblizkova M, Klanova J (2010) Calibration of silicone rubber passive samplers: experimental and modeled relations between sampling rate and compound properties. Environ Sci Technol 44:362–367
Acknowledgments
The study was funded by SNF project 200021–121738.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 5052 kb)
Rights and permissions
About this article
Cite this article
Vermeirssen, E.L.M., Dietschweiler, C., Escher, B.I. et al. Uptake and release kinetics of 22 polar organic chemicals in the Chemcatcher passive sampler. Anal Bioanal Chem 405, 5225–5236 (2013). https://doi.org/10.1007/s00216-013-6878-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-6878-1