Abstract
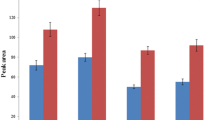
A method using microextraction by packed sorbent (MEPS) and gas chromatography–tandem mass spectrometry (GC-MS/MS) is described for the determination of seven antipsychotic drugs in human plasma. The studied compounds were chlorpromazine (CPZ), haloperidol (HAL), cyamemazine, quetiapine, clozapine, olanzapine (OLZ), and levomepromazine; promazine, protriptyline, and deuterated CPZ were used as internal standards. The validation parameters included selectivity, linearity and limits of detection and quantitation, intra- and interday precision and trueness, recovery, and stability and were studied according to internationally accepted guidelines. The method was found to be linear between the lower limit of quantitation and 1000 ng/mL, except for OLZ and HAL (200 ng/mL), with determination coefficients higher than 0.99 for all analytes, and extraction efficiencies ranged from 62 to 92 %. Intra- and interday precision ranged from 0.24 to 10.67 %, while trueness was within a ±15 % interval from the nominal concentration for all analytes at all studied levels. MEPS has shown to be a rapid procedure for the determination of the selected antipsychotic drugs in human plasma, allowing reducing the handling time and the costs of analysis. Furthermore, GC-MS/MS has demonstrated to be a powerful tool for the simultaneous quantitation of the studied compounds, enabling obtaining adequate selectivity and sensitivity using a sample volume of as low as 0.25 mL.



Similar content being viewed by others
References
Rao LV, Snyder ML, Vallaro GM (2009) Rapid liquid chromatography/tandem mass spectrometer (LCMS) method for clozapine and its metabolite N-desmethyl clozapine (Norclozapine) in human serum. J Clin Lab Anal 23:394–398
Saar E, Gerostamoulos D, Drummer OH, Beyer J (2010) Identification and quantification of 30 antipsychotics in blood using LC-MS/MS. J Mass Spectrom 45:915–925
Zhang G, Terry AV Jr, Bartlett MG (2008) Bioanalytical methods for the determination of antipsychotic drugs. Biomed Chromatogr 22:671–687
Zhang G, Terry AV Jr, Bartlett MG (2007) Simultaneous determination of five antipsychotic drugs in rat plasma by high performance liquid chromatography with ultraviolet detection. J Chromatogr B 856:20–28
Mercolini L, Grillo M, Bartoletti C, Boncompagni G, Raggi MA (2007) Simultaneous analysis of classical neuroleptics, atypical antipsychotics and their metabolites in human plasma. Anal Bioanal Chem 388:235–243
Mercolini L, Bugamelli F, Kenndler E, Boncompagni G, Franchini L, Raggi MA (2007) Simultaneous determination of the antipsychotic drugs levomepromazine and clozapine and their main metabolites in human plasma by a HPLC-UV method with solid-phase extraction. J Chromatogr B 846:273–280
Cutroneo P, Beljean M, Luu RP, Siouffi AM (2006) Optimization of the separation of some psychotropic drugs and their respective metabolites by liquid chromatography. J Pharm Biomed Anal 41:333–340
Bazhdanzadeh S, Talebpour Z, Adib N, Aboul-Enein HY (2011) A simple and reliable stir bar sorptive extraction-liquid chromatography procedure for the determination of chlorpromazine and trifluoperazine in human serum using experimental design methodology. J Sep Sci 34:90–97
Kirchherr H, Kühn-Velten WN (2006) Quantitative determination of forty-eight antidepressants and antipsychotics in human serum by HPLC tandem mass spectrometry: a multi-level, single-sample approach. J Chromatogr B 843:100–113
de la Torre C, Martínez MA, Almarza E (2005) Determination of several psychiatric drugs in whole blood using capillary gas–liquid chromatography with nitrogen phosphorus detection: comparison of two solid phase extraction procedures. Forensic Sci Int 155:193–204
Vardakou I, Dona A, Pistos C, Alevisopoulos G, Athanaselis S, Maravelias C, Spiliopoulou C (2010) Validated GC/MS method for the simultaneous determination of clozapine and norclozapine in human plasma. Application in psychiatric patients under clozapine treatment. J Chromatogr B 878:2327–2332
Saracino MA, Lazzara G, Prugnoli B, Raggi MA (2011) Rapid assays of clozapine and its metabolites in dried blood spots by liquid chromatography and microextraction by packed sorbent procedure. J Chromatogr A 1218:2153–2159
Choong E, Rudaz S, Kottelat A, Guillarme D, Veuthey JL, Eap CB (2009) Therapeutic drug monitoring of seven psychotropic drugs and four metabolites in human plasma by HPLC-MS. J Pharm Biomed Anal 50:1000–1008
Hasselstrøm J (2011) Quantification of antidepressants and antipsychotics in human serum by precipitation and ultra high pressure liquid chromatography–tandem mass spectrometry. J Chromatogr B 879:123–128
Tanaka E, Nakamura T, Terada M, Shinozuka T, Hashimoto C, Kurihara K, Honda K (2007) Simple and simultaneous determination for 12 phenothiazines in human serum by reversed-phase high-performance liquid chromatography. J Chromatogr B 854:116–120
Rosland M, Szeto P, Procyshyn R, Barr AM, Wasan KM (2007) Determination of clozapine and its metabolite, norclozapine in various biological matrices using high-performance liquid chromatography. Drug Dev Ind Pharm 33:1158–1166
Sachse J, Köller J, Härtter S, Hiemke C (2006) Automated analysis of quetiapine and other antipsychotic drugs in human blood by high performance-liquid chromatography with column-switching and spectrophotometric detection. J Chromatogr B 830:342–348
Li J, Zhao F, Ju H (2006) Simultaneous determination of psychotropic drugs in human urine by capillary electrophoresis with electrochemiluminescence detection. Anal Chim Acta 575:57–61
Lara FJ, García-Campaña AM, Alés-Barrero F, Bosque-Sendra JM (2005) Development and validation of a capillary electrophoresis method for the determination of phenothiazines in human urine in the low nanogram per milliliter concentration range using field-amplified sample injection. Electrophoresis 26:2418–2429
Saar E, Beyer J, Gerostamoulos D, Drummer OH (2012) The analysis of antipsychotic drugs in human matrices using LC-MS(/MS). Drug Test Anal 4:376–394
Zhang G, Terry AV Jr, Bartlett MG (2007) Sensitive liquid chromatography/tandem mass spectrometry method for the simultaneous determination of olanzapine, risperidone, 9-hydroxyrisperidone, clozapine, haloperidol and ziprasidone in rat brain tissue. J Chromatogr B 858:276–281
Zhang G, Terry AV Jr, Bartlett MG (2007) Sensitive liquid chromatography/tandem mass spectrometry method for the determination of the lipophilic antipsychotic drug chlorpromazine in rat plasma and brain tissue. J Chromatogr B 854:68–76
Niederländer HA, Koster EH, Hilhorst MJ, Metting HJ, Eilders M, Ooms B, de Jong GJ (2006) High throughput therapeutic drug monitoring of clozapine and metabolites in serum by on-line coupling of solid phase extraction with liquid chromatography–mass spectrometry. J Chromatogr B 834:98–107
de Castro A, Fernandez MMR, Laloup M, Samyn N, Boeck G, Wood M, Maes V, López-Rivadulla M (2007) High-throughput on-line solid-phase extraction–liquid chromatography–tandem mass spectrometry method for the simultaneous analysis of 14 antidepressants and their metabolites in plasma. J Chromatogr A 1160:3–12
Moreno IED, da Fonseca BM, Barroso M, Costa S, Queiroz JA, Gallardo E (2012) Determination of piperazine-type stimulants in human urine by means of microextraction in packed sorbent and high performance liquid chromatography–diode array detection. J Pharm Biomed Anal 61:93–99
Miyaguchi H, Iwata Y, Kanamori T, Tsujikawa K, Kuwayama K, Inoue H (2009) Rapid identification and quantification of methamphetamine and amphetamine in hair by gas chromatography/mass spectrometry coupled with micropulverized extraction, aqueous acetylation and microextraction by packed sorbent. J Chromatogr A 1216:4063–4070
Moreno IED, da Fonseca BM, Magalhães AR, Geraldes VS, Queiroz JA, Barroso M, Costa S, Gallardo E (2012) Rapid determination of piperazine-type stimulants in human urine by microextraction in packed sorbent after method optimization using a multivariate approach. J Chromatogr A 1222:116–120
Abdel-Rehim M, Altun Z, Blomberg L (2004) Microextraction in packed syringe (MEPS) for liquid and gas chromatographic applications. Part II—determination of ropivacaine and its metabolites in human plasma samples using MEPS with liquid chromatography/tandem mass spectrometry. J Mass Spectrom 39:1488–1493
Abdel-Rehim M (2010) Recent advances in microextraction by packed sorbent for bioanalysis. J Chromatogr A 1217:2569–2580
Chaves A, Leandro F, Carris J, Queiroz ME (2010) Microextraction in packed sorbent for analysis of antidepressants in human plasma by liquid chromatography and spectrophotometric detection. J Chromatogr B 878:2123–2129
Saracino MA, de Palma A, Boncompagni G, Raggi MA (2010) Analysis of risperidone and its metabolite in plasma and saliva by LC with coulometric detection and a novel MEPS procedure. Talanta 81:1547–1553
International Conference on Harmonization (ICH), Validation of Analytical Procedures: Methodology ICH Q2 B. (2005). Available from http://www.ich.org/LOB/media/MEDIA417.pdf. Accessed 29 November 2011
U.S. Department of Health and Human Services, Food and Drug Administration, Guidance for Industry: Bioanalytical Method Validation (2001). Available from http://www.fda.gov/downloads/Drugs/GuidanceCompilanceRegulatoryInformation/Guidances/ucm070107.pdf. Accessed 29 November 2011
World Anti-doping Agency, International standard for laboratories: identification criteria for qualitative assays incorporating column chromatography and mass spectrometry (2010). Available from http://www.wadaama.org/Documents/World_AntiDoping_Program/WADP-IS-Laboratories/WADA_TD2010IDCRv1.0_IdentificationCriteriaforQualitativeAssays_May082010_EN.doc.pdf. Accessed 3 January 2012
Barroso M, Costa S, Dias M, Vieira DN, Queiroz JA, López-Rivadulla M (2010) Analysis of phenylpiperazine-like stimulants in human hair as trimethylsilyl derivatives by gas chromatography–mass spectrometry. J Chromatogr A 1217:6274–6280
Saar E, Gerostamoulos D, Drummer OH, Beyer J (2012) Identification of 2-hydroxymethyl-olanzapine as a novel degradation product of olanzapine. Forensic Sci Int 220:74–79
Acknowledgments
The authors acknowledge the Protocol UBI/Santander-Totta in the form of two fellowships (Ivo Moreno and Beatriz da Fonseca), the program COMPETE and the Portuguese Foundation for Science and Technology (PEst-C/SAU/UI0709/2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special paper collection Forensic Toxicology with guest editors Kazuhito Watanabe and Satoshi Chinaka.
Rights and permissions
About this article
Cite this article
da Fonseca, B.M., Moreno, I.E.D., Barroso, M. et al. Determination of seven selected antipsychotic drugs in human plasma using microextraction in packed sorbent and gas chromatography–tandem mass spectrometry. Anal Bioanal Chem 405, 3953–3963 (2013). https://doi.org/10.1007/s00216-012-6695-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6695-y