Abstract
Two synthetic precursor peptides, H2N-CVGIW and H2N-LVMCCVGIW, involved in the quorum sensing of Lactobacillus plantarum WCFS1, were characterized by mass spectrometry (MS) with electrospray ionization and 7-T Fourier transform ion cyclotron resonance (ESI-FTICR) instrument. Cell-free bacterial supernatant solutions were analyzed by reversed-phase liquid chromatography with ESI-FTICR MS to verify the occurrence of both pentapeptide and nonapeptide in the bacterial broth. The structural characterization of both protonated peptides was performed by infrared multiphoton dissociation using a continuous CO2 laser source at a wavelength of 10.6 μm. As their fragmentation behavior cannot be directly derived from the primary peptide structure, all anomalous fragments were interpreted as neutral loss of amino acids from the interior of both peptides, i.e., loss of V, G, VG and M, MC, V, CC, from H2N-CVGIW and H2N-LVMCCVGIW, respectively. Mechanisms of this scrambling are proposed. FTICR MS provides accurate masses of all fragment ions with very low absolute mass errors (<1.6 ppm), which facilitated the reliable assignment of their elemental compositions. The resolving power was more than sufficient to resolve closely isobaric product ions with routine subparts per million mass accuracies. Only the occurrence of pentapeptide was found in the cell-free culture of L. plantarum, grown in Waymouth’s medium broth, with a low content of 5.2 ± 2.6 μM by external calibration. Most of it was present as oxidized H2N-CVGIW, that is, the soluble disulfide pentapeptide with a level tenfold higher (i.e., 50 ± 4 μM, n = 3).

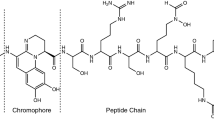
IRMPD of the precursor protonated peptide, [H2N-CVGIW +H]+ at m/z 577.3 and suggested pathway showing the formation of peptide macrocycle and its selective ring opening.






Similar content being viewed by others
References
Winston RL, Fitzgerald MC (1997) Mass spectrometry as a readout of protein structure and function. Mass Spectrom Rev 16:165–179
Godovac-Zimmermann J, Brown LR (2001) Perspectives for mass spectrometry and functional proteomics. Mass Spectrom Rev 20:1–57
Paizs B, Suhal S (2005) Fragmentation pathways of protonated peptides. Mass Spectrom Rev 24:508–548
Karas M, Bahr U (1990) Laser desorption ionization mass spectrometry of large biomolecules. Trends Anal Chem 9:321–325
Kalkum M, Lyon GJ, Chait BT (2003) Detection of secreted peptides by using hypothesis-driven multistage mass spectrometry. PNAS 100:2795–2800
Villanueva J, Philip J, Entenberg D, Chaparro CA, Tanwar MK, Holland EC, Tempst P (2004) Serum peptide profiling by magnetic particle-assisted, automated sample processing and MALDI-TOF mass spectrometry. Anal Chem 76:1560–1570
Batoy S, Akhmetova E, Miladinovic S, Smeal J, Wilkins CL (2008) Developments in MALDI mass spectrometry: the quest for the perfect matrix. Appl Spectrosc Rev 43:485–550
Hop C, Bakhtiar R (1997) An introduction to electrospray ionization and matrix-assisted laser desorption/ionization mass spectrometry: essential tools in a modern biotechnology environment. Biospectroscopy 3:259–280
Martin SE, Shabanowitz J, Hunt DF, Marto JA (2000) Subfemtomole MS and MS/MS peptide sequence analysis using nano-HPLC micro-ESI Fourier transform ion cyclotron resonance mass spectrometry. Anal Chem 72:4266–4274
Hu Q, Noll RJ, Li H, Makarov A, Hardman M, Graham Cooks R (2005) The Orbitrap: a new mass spectrometer. J Mass Spectrom 40:430–443
Todd JFJ, March RE (2005) Quadrupole ion trap mass spectrometry, 2nd edn. Wiley & Sons, Hoboken
Douglas DJ, Frank AJ, Mao D (2005) Linear ion traps in mass spectrometry. Mass Spectrom Rev 24:1–29
Wollnik H (1993) Time-of-flight mass analyzers. Mass Spectrom Rev 12:89–114
Chernushevich IV, Loboda AV, Thomson BA (2001) An introduction to quadrupole-time-of-flight mass spectrometry. J Mass Spectrom 36:849–865
He F, Emmett MR, Håkansson K, Hendrickson CL, Marshall AG (2004) Theoretical and experimental prospects for protein identification based solely on accurate mass measurement. J Proteom Res 3:61–67
Marshall AG, Hendrickson CL, Jackson GS (1998) Fourier transform ion cyclotron resonance mass spectrometry: a primer. Mass Spectrom Rev 17:1–35
Marshall AG (2000) Milestones in Fourier transform ion cyclotron resonance mass spectrometry technique development. Int J Mass Spectrom 200:331–356
Vachet RW, Bishop BM, Erickson BW, Glish GL (1997) Novel peptide dissociation: gas-phase intramolecular rearrangement of internal amino acid residues. J Am Chem Soc 119:5481–5488
Tang XJ, Thibault P, Boyd RK (1993) Fragmentation reactions of multiply-protonated peptides and implications for sequencing by tandem mass spectrometry with low-energy collision-induced dissociation. Anal Chem 65:2824–2834
Tang XJ, Boyd RK (1994) Rearrangements of doubly charged acylium ions from lysyl and ornithyl peptides. Rapid Commun Mass Spectrom 8:678–686
Yague J, Paradela A, Ramos M, Ogueta S, Marina A, Barahona F, Lopez de Castro JA, Vazquez J (2003) Peptide rearrangement during quadrupole ion trap fragmentation: added complexity to MS/MS spectra. Anal Chem 75:1524–1535
Harrison AG, Young AB, Christian B, Suhai S, Paizs B (2006) Scrambling of sequence information in collision-induced dissociation of peptides. J Am Chem Soc 128:10364–10365
Jia C, Qi W, He Z (2007) Cyclization reaction of peptide fragment ions during multistage collisionally activated decomposition: an inducement to lose internal amino-acid residues. J Am Soc Mass Spectrom 18:663–678
Bleiholder C, Osburn S, Williams TD, Suhai S, Van Stipdonk M, Harrison AG, Paizs B (2008) Sequence-scrambling fragmentation pathways of protonated peptides. J Am Chem Soc 130:17774–17789
Saminathan IS, Wang XS, Guo Y, Krakovska O, Voisin S, Hopkinson AC, Siu KWM (2010) The extent and effects of peptide sequence scrambling via formation of macrocyclic b ions in model proteins. J Am Soc Mass Spectrom 21:2085–2094
Sturme MHJ, Nakayama J, Molenaar D, Murakami Y, Kleerebezem M, de Vos M (2005) An agr-like two-component regulatory system in Lactobacillus plantarum is involved in production of a novel cyclic peptide and regulation of adherence. J Bacteriol 187:5224–5235
Fridgen TD, McMahon TB (2005) IRMPD. In: Gross ML, Caprioli RM (eds) The encyclopedia of mass spectrometry, vol. 4. Elsevier, Oxford
Cooper HJ (2010) Fourier transform ion cyclotron resonance mass spectrometry in the analysis of peptides and proteins. In: March RE, Todd JFJ (eds) Spectroscopy of ions stored in trapping mass spectrometers in practical aspects of trapped ion mass spectrometry, Volume V: Applications of Ion Trapping Devices, CRC Press, Taylor and Francis: Boca Raton
McFarland MA, Marshall AG, Hendrickson CL, Nilsson CL (2005) Structural characterization of the GM1 ganglioside by infrared multiphoton dissociation, electron capture dissociation, and electron detachment dissociation electrospray ionization FT-ICR MS/MS. J Am Soc Mass Spectrom 16:752–762
Polfer NC (2011) Infrared multiple photon dissociation spectroscopy of trapped ions. Chem Soc Rev 40:2211–2221
Roepstorff P, Fohlmann J (1984) Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed Mass Spectrom 11:601–601
Madsen JA, Brodbelt JS (2009) Comparison of infrared multiphoton dissociation and collision-induced dissociation of supercharged peptides in ion traps. J Am Soc Mass Spectrom 20:349–358
Yalcin T, Csizmadia IG, Peterson MR, Harrison AG (1996) The structure and fragmentation of B n (n ≥ 3) ions in peptide spectra. J Am Soc Mass Spectrom 7:233–242
Yalcin T, Khouw C, Csizmadia IG, Peterson MR, Harrison AG (1995) Why are B ions stable species in peptide spectra? J Am Soc Mass Spectrom 6:1165–1174
Garcia IR, Giles K, Bateman RH, Gaskell SJ (2008) Studies of peptide a- and b-type fragment ions using stable isotope labeling and integrated ion mobility/tandem mass spectrometry. J Am Soc Mass Spectrom 19:1781–1787
Steen H, Mann M (2004) The abc’s (and xyz’s) of peptide sequencing. Nat Rev Mol Cell Biol 5:699–711
Paizs B, Suhai S (2005) Fragmentation pathways of protonated peptides. Mass Spectrom Rev 24:508–548
Bythell BJ, Suhai S, Somogyi A, Paizs B (2009) Proton-driven amide bond-cleavage pathways of gas-phase peptide ions lacking mobile protons. J Am Chem Soc 131:14057–14065
Bythell BJ, Maitre P, Paizs B (2010) Cyclization and rearrangement reactions of a(n) fragment ions of protonated peptides. J Am Chem Soc 132:14766–14779
Wysocki VH, Resing KA, Zhang Q, Cheng G (2005) Mass spectrometry of peptides and proteins. Methods 35:211–222
Zou S, Oomens J, Polfer NC (2012) Competition between diketopiperazine and oxazolone formation in water loss products from protonated ArgGly and GlyArg. Int J Mass Spectrom 316:12–17
Gucinski AC, Chamot-Rooke J, Nicol E, Somogyi A, Wysocki VH (2012) Structural influences on preferential oxazolone versus diketopiperazine b2+ ion formation for histidine analogue-containing peptides. J Phys Chem A 116:4296–4304
Mathur R, O’Connor PB (2009) Artifacts in Fourier transform mass spectrometry. Rapid Comm Mass Spectrom 23(4):523–529
Cataldi TRI, Bianco G, Abate S (2009) Accurate mass analysis of N-acyl-homoserine-lactones and cognate lactone-opened compounds in bacterial isolates of Pseudomonas aeruginosa PAO1 by LC-ESI-LTQ-FTICR-MS. J Mass Spectrom 44:182–192
Zhang L, Gray L, Novick RP, Ji G (2002) Transmembrane topology of AgrB, the protein Involved in the post-translational modification of AgrD in Staphylococcus aureus. J Biol Chem 277:34736–34742
Sturme MH, Kleerebezem M, Nakayama J, Akkermans AD, Vaugha EE, de Vos WM (2002) Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie Van Leeuwenhoek 81:233–243
Muskal SM, Holbrook SR, Kim SH (1990) Prediction of the disulfide-bonding state of cysteine in proteins. Protein Engineering 11:667–672
Mucchielli-Giorgi MH, Hazout S, Tuffery P (2002) Predicting the disulfide bonding state of cysteines using protein descriptors. Proteins 46:243–249
Lioe H, O’Hair RAJ (2007) A novel salt bridge mechanism highlights the need for nonmobile proton conditions to promote disulfide bond cleavage in protonated peptides under low-energy collisional activation. J Am Soc Mass Spectrom 18:1109–1123
Mihalca R, van der Burgt YE, Heck AJ, Heeren RM (2007) Disulfide bond cleavages observed in SORI-CID of three nonapeptides complexed with divalent transition-metal cations. J Mass Spectrom 42(4):450–458
Gauthier JW, Trautman TR, Jacobson DB (1991) Sustained off-resonance irradiation for CAD involving FTMS. CAD technique that emulates infrared multiphoton dissociation. Anal Chim Acta 246:211–225
McLuckey SA, Goeringer DE (1997) Slow heating methods in tandem mass spectrometry. J Mass Spectrom 35:461–474
Barrow MP, Burkitt WI, Derrick PJ (2005) Principles of Fourier transform ion cyclotron resonance mass spectrometry and its application in structural biology. Analyst 130:18–28
Seipert RR, Dodds ED, Clowers BH, Beecroft SM, German JB, Lebrilla CB (2008) Factors that influence fragmentation behavior of N-linked glycopeptide ions. Anal Chem 80:3684–3692
Coffinier Y, Nguyen N, Drobecq H, Melnyk O, Thomy V, Boukherroub R (2012) Affinity surface-assisted laser desorption/ionization mass spectrometry for peptide enrichment. Analyst 137:5527–5532
Gao Y, Wang Y (2007) A method to determine the ionization efficiency change of peptides caused by phosphorylation. J Am Soc Mass Spectrom 18:1973–1976
Acknowledgments
The authors gratefully acknowledge Prof. Eugenio Parente (University of Basilicata, Potenza, Italy) for providing the culture broth of L. plantarum strain WCFS1. This work was performed using the instrumental facilities of CIGAS Center founded by the EU (project no. 2915/12), Regione Basilicata, and Università degli Studi della Basilicata. We are also grateful to Domenico Montesano for his technical support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1180 kb)
Rights and permissions
About this article
Cite this article
Bianco, G., Labella, C., Pepe, A. et al. Scrambling of autoinducing precursor peptides investigated by infrared multiphoton dissociation with electrospray ionization and Fourier transform ion cyclotron resonance mass spectrometry. Anal Bioanal Chem 405, 1721–1732 (2013). https://doi.org/10.1007/s00216-012-6583-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6583-5