Abstract
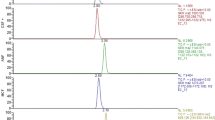
Azole antifungal drugs are important in the prophylaxis and treatment of invasive aspergillosis. Therapeutic drug monitoring may be indicated to (1) monitor adherence, (2) guide dosage and (3) minimise the risk of drug–drug interactions and dose-related toxicity. TurboFlowTM technology offers online, automated sample preparation. An Aria TranscendTM TLX-II coupled with a TSQ VantageTM MS was used. Centrifuged samples (25 μL) were mixed with internal standard solution (975 μL) and 30 μL injected directly onto a C18-P-XL TurboFlow column. Analytes were focussed onto a Phenomenex Gemini Phenyl analytical column and eluted using a methanol/water gradient (flow-rate, 0.8 mL/min). Analytes were monitored in selected reaction monitoring mode (two transitions per analyte, positive mode APCI). Calibration ranges were as follows: itraconazole, hydroxyitraconazole, and posaconazole 0.05–5.0 mg/L; voriconazole and fluconazole 0.1–10 mg/L. Total analysis time was 12 min. TurboFlow column recovery was >77% for all analytes. Calibration was linear (R 2 > 0.99) for all analytes. Inter- and intra-assay imprecision (% RSD) was <8% and accuracy (nominal internal quality control values) 90–105% for all analytes. The limit of detection was 0.01 mg/L for all analytes. No matrix effects were observed. This method is simple, robust and suitable for measuring these compounds at concentrations attained during therapy.



Similar content being viewed by others
References
Bow E, Loewen R, Cheang M, Schacter B (1995) Invasive fungal disease in adults undergoing remission-induction therapy for acute myeloid leukaemia: the pathogenic role of the antileukaemic regimen. Clin Inf Dis 21:361–369
Bow E (1998) Infection risk and cancer chemotherapy: the impact of the chemotherapeutic regimen in patients with lymphoma and solid tissue malignancies. J Antimicrob Chemother 41:1–5
Maertens J (2007) Evaluating prophylaxis if invasive fungal infections in patients with haematological malignancies. Eur J Haematol 78:275–282
Cornely O, Bohme A, Buchheidt D et al (2009) Primary prophylaxis of invasive fungal infections in patients with haematologic malignancies. Haematologica 94:113–122
Groll A, De Lucca A, Walsh T (1998) Emerging targets for the development of novel antifungal therapeutics. Trends Microbiol 6:117–124
Heimark L, Shipkova P, Greene J et al (2002) Mechanism of azole antifungal activity as determined by liquid chromatographic/mass spectrometric monitoring of ergosterol biosynthesis. J Mass Spectrom 37:265–269
Lewis RE (2011) Pharmacokinetic-pharmacodynamic optimization of triazole antifungal therapy. Curr Opin Infect Dis 24(Suppl 2):S14–S29
Hope W, Billaud E, Lestner J, Denning D (2008) Therapeutic drug monitoring for triazoles. Curr Opin Infect Dis 21:580–586
Goodwin ML, Drew RH (2008) Antifungal serum concentration monitoring: an update. J Antimicrob Chemother 61:17–25
Andes D, Pascual A, Marchetti O (2009) Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob Agents Chemother 53:24–34
Lestner J, Roberts S, Moore C, Howard S, Denning D, Hope W (2009) Toxicodynamics of itraconazole: implications for therapeutic drug monitoring. Clin Infect Dis 49:928–930
Hostetler JS, Heykants J, Clemons K, Woestenborghs R, Hanson LH, Stevens DA (1993) Discrepancies in bioassay and chromatography determinations explained by metabolism of itraconazole to hydroxyitraconazole: studies of interpatient variations in concentrations. Antimicrob Agents Chemother 37:2224–2227
Purkins L, Wood N, Ghahramani P et al (2002) Pharmacokinetics and safety of voriconazole following intravenous- to oral-dose escalation regimens. Antimicrob Agents Chemother 46:2546–2553
Brüggemann RJ, Donnelly JP, Aarnoutse RE et al (2008) Therapeutic drug monitoring of voriconazole. Ther Drug Monit 30:403–411
Gubbins P, Krishna G, Sansone-Parsons A et al (2006) Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob Agents Chemother 50:1993–1999
Ullmann A, Cornely O, Burchardt A et al (2006) Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob Agents Chemother 50:658–666
Krishna G, Martinho M, Chandrasekar P, Ullmann A, Patino H (2007) Pharmacokinetics of oral posaconazole in allogenic haematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27:1627–1636
Glasmacher A, Hahn C, Leutner C et al (1999) Breakthrough invasive fungal infections in patients after prophylaxis with itraconazole. Mycoses 42:443–451
Smith J, Safdar N, Knasinski V et al (2006) Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother 50:1570–1572
Pascual A, Calandra T, Bolay S et al (2008) Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis 46:201–211
Edge T (2003) Turbulent flow chromatography in bioanalysis. In: Wilson ID (ed) Handbook of analytical separations, vol 4. Elsevier Science B.V, Amsterdam, pp 91–128
Morgan PE, Couchman L, Robinson S, McDonnell S, Flanagan RJ (2010) Analysis of clozapine and norclozapine in plasma using on-line sample preparation and LC-MS/MS. The Column 6:10–16
Couchman L, Nooijen P, Birch M, Robinson S, Flanagan RJ (2010) Simultaneous and sensitive analysis of dasatinib, imatinib, norimatinib and nilotinib in human plasma using TurboFlow LC-MS/MS. Toxichem Krimtech 77:218
Chahbouni A, Wilhelm AJ, den Burger JC, Sinjewel A, Vos RM (2010) Validated liquid chromatography-tandem mass spectroscopy method for the simultaneous quantification of four antimycotic agents in human serum. Ther Drug Monit 32:453–457
Alffenaar JWC, Wessels A, van Hateren K, Greijdanus B, Kosterink J, Uges D (2010) Method for therapeutic drug monitoring of azole antifungal drugs in human serum using LC/MS/MS. J Chromatogr B 878:39–44
Ramos L, Brignol N, Bakhtiar R, Ray T, McMahon LM, Tse FL (2000) High-throughput approaches to the quantitative analysis of ketoconazole, a potent inhibitor of cytochrome P450 3A4, in human plasma. Rapid Commum Mass Spectrom 14:2282–2293
FDA/CDER (Food and Drug Administration/Center for Drug Evaluation and Research) (2001) Guidance for industry. Bioanalytical method validation. See http://www.fda.gov/cder/guidance/4252fnl.htm (last checked 5 May 2009)
Bonfiglio R, King RC, Olah TV, Merkle K (1999) The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun Mass Spectrom 13:1175–1185
Couchman L, Birch M, Ireland R et al (2012) An automated method for the measurement of a range of tyrosine kinase inhibitors in human plasma or serum using turbulent flow liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 403:1685–1695
Buckner SL, Ceesay MM, Pagliuca A, Morgan PE, Flanagan RJ (2011) Measurement of posaconazole, itraconazole and hydroxyitraconazole in plasma/serum by HPLC with fluorescence detection. Ther Drug Monit 33:735–741
Boogaerts M, Verhoef G, Zachee P, Demuuynck H, Verbist L, de Beule K (1989) Antifungal prophylaxis with itraconazole in prolonged neutropenia: correlation with plasma levels. Mycoses 32:103–108
Hardin T, Graybill J, Fetchick R, Woestenborghs R, Rinaldi M, Kuhn J (1988) Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob Agents Chemother 32:1310–1313
FDA/CDER (2009) Posaconazole. FDA briefing document. See http://www.fda.gov/cder/foi/nda/2006/022003s000_NoxafilTOC.htm (last checked 27 July 2009)
Thomson G, Rinaldi M, Patterson T, Lewis J (2009) Posaconazole therapeutic drug monitoring: a reference laboratory experience. Antimicrob Agents Chemother 53:2223–2224
Lebeaux D, Lanternier F, Elie C et al (2009) Therapeutic drug monitoring of posaconazole: a monocentric study with 54 adults. Antimicrob Agents Chemother 53:5224–5229
Matsumoto K, Ikawa K, Abematsu K et al (2009) Correlation between voriconazole trough plasma concentration and hepatotoxicity in patients with different CYP2C19 genotypes. Int J Antimicrob Agents 34:91–94
Acknowledgments
Thanks to Tom Whitehouse and Jeff Zonderman (ThermoFisher Scientific) for analytical support, to Mr. A. Noel and Dr. M. Darville from the UKNEQAS Antifungal Scheme and to Prof. D. Uges from the KKGT EQA Scheme for permission to cite data from their schemes. Thanks also to Janssen-Cilag and to Pfizer for the gift of reference materials and to Pfizer and Gilead for sponsoring the Aspergillosis study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Couchman, L., Buckner, S.L., Morgan, P.E. et al. An automated method for the simultaneous measurement of azole antifungal drugs in human plasma or serum using turbulent flow liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem 404, 513–523 (2012). https://doi.org/10.1007/s00216-012-6176-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6176-3