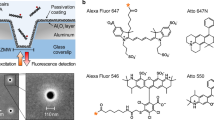
Abstract
Functional surfaces and especially the control of surface properties depending on external parameters such as light illumination have gained increasing importance in the last few years. We present the characterization of polymers from the cycloolefin (co)polymer class (COC/COP) functionalized with an aminosilane as a basis for the further immobilization of compounds. In a first step, an assay using AlexaFluor®647 fluorescent dye was used to assess surface homogeneity and reproducibility. A coefficient of variation of less than 15% for dot-to-dot and less than 25% for chip-to-chip could be achieved. The same amino-functionalized surfaces were then used to immobilize a biotinylated photolabile linker compound, binding AlexaFluor®647-labeled streptavidin. The linker was photocleaved with high efficiency at λ = 365 nm and P = 0.15 mW/cm2. Fluorescence measurements show that polymers of the COC/COP class can be used as versatile surfaces for the photoinduced release of compounds immobilized via photolabile linkers.

Photo-induced release of a model compound



Similar content being viewed by others
References
Kantevari S, Hoang CJ, Ogrodnik J, Egger M, Niggli E, Ellis-Davies GCR (2006) ChemBioChem 7:174–180
Dell'Aquila C, Imbach J-L, Rayner B (1997) Tetrahedron Lett 38:5289–5292
Dormán G, Prestwich GD (2000) Trends Biotechnol 18:64–77
Du LH, Zhang SJ, Wang YG (2005) Tetrahedron Lett 46:3399–3402
Seo TS, Bai X, Kim DH, Meng Q, Shi S, Ruparel H, Li Z, Turro NJ, Ju J (2005) Proc Natl Acad Sci 102:5926–5931
Timko BP, Dvir T, Kohane DS (2010) Adv Mater 22:4925–4943
Bai X, Li Z, Jockusch S, Turro NJ, Ju J (2003) Proc Natl Acad Sci 100:409–413
Holmes CP, Jones DG (1995) J Org Chem 60:2318–2319
Kim MS, Diamond SL (2006) Bioorg Med Chem Lett 16:4007–4010
Kim MS, Gruneich J, Jinga H, Diamond SL (2010) J Mater Chem 20:3396–3403
Rodebaugh R, Fraser-Reid B, Geysen HM (1997) Tetrahedron Lett 38:7653–7656
Yan F, Chen L, Tang Q, Wang R (2004) Bioconjug Chem 15:1030–1036
Barth A, von Germar F, Kreutz W, Mäntele W (1996) J Biol Chem 271:30637–30646
del Campo A, Boos D, Spiess HW, Jonas U (2005) Angew Chem Int Ed 44:4707–4712
Alonso JM, Reichel A, Piehler J, del Campo A (2008) Langmuir 24:448–457
Bochet CG (2002) J Chem Soc Perkin Trans 1:125–142
Ahn CH, Choi J-W, Beaucage G, Nevin JH, Lee J-B, Puntambekar A, Lee JY (2004) Proc IEEE 92:154–173
Brandenburg A, Curdt F, Sulz G, Ebling F, Nestler J, Wunderlich K, Michel D (2009) Sens Actuators B 139:245–251
Jönsson C, Aronsson M, Rundström G, Pettersson C, Mendel-Hartvig I, Bakker J, Martinsson E, Liedberg B, MacCraith B, Öhman O, Melin J (2008) Lab Chip 8:1191–1197
Goddard JM, Hotchkiss JH (2007) Prog Polym Sci 32:698–725
Hwang S, Tseng M, Shu J, Yu HH (2008) Surf Coat Technol 202:3669–3674
Acknowledgments
We acknowledge financial support from the European Community in the framework of the Eurostars Programme and Rhenovia Pharma. We are grateful for the assistance during surface analytical measurements provided by Nadja Ehrhardt and Thomas Trutschel, Institute for Bioprocessing and Analytical Measurement Techniques.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmitt, K., Rist, J. & Hoffmann, C. Optical waveguides for the evanescent wave-induced cleavage of photolabile linker compounds. Anal Bioanal Chem 401, 777–782 (2011). https://doi.org/10.1007/s00216-011-5086-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5086-0