Abstract
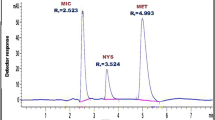
The purpose of this study was the development and validation of an LC–MS–MS method for simultaneous analysis of ibuprofen (IBP), 2-hydroxyibuprofen (2-OH-IBP) enantiomers, and carboxyibuprofen (COOH-IBP) stereoisomers in fungi culture medium, to investigate the ability of some endophytic fungi to biotransform the chiral drug IBP into its metabolites. Resolution of IBP and the stereoisomers of its main metabolites was achieved by use of a Chiralpak AS-H column (150 × 4.6 mm, 5 μm particle size), column temperature 8 °C, and the mobile phase hexane–isopropanol–trifluoroacetic acid (95: 5: 0.1, v/v) at a flow rate of 1.2 mL min−1. Post-column infusion with 10 mmol L−1 ammonium acetate in methanol at a flow rate of 0.3 mL min−1 was performed to enhance MS detection (positive electrospray ionization). Liquid–liquid extraction was used for sample preparation with hexane–ethyl acetate (1:1, v/v) as extraction solvent. Linearity was obtained in the range 0.1–20 μg mL−1 for IBP, 0.05–7.5 μg mL−1 for each 2-OH-IBP enantiomer, and 0.025–5.0 μg mL−1 for each COOH-IBP stereoisomer (r ≥ 0.99). The coefficients of variation and relative errors obtained in precision and accuracy studies (within-day and between-day) were below 15%. The stability studies showed that the samples were stable (p > 0.05) during freeze and thaw cycles, short-term exposure to room temperature, storage at −20 °C, and biotransformation conditions. Among the six fungi studied, only the strains Nigrospora sphaerica (SS67) and Chaetomium globosum (VR10) biotransformed IBP enantioselectively, with greater formation of the metabolite (+)-(S)-2-OH-IBP. Formation of the COOH-IBP stereoisomers, which involves hydroxylation at C3 and further oxidation to form the carboxyl group, was not observed.

Enantioselective biotransformation of ibuprofen to 2-hydroxyibuprofen by endophytic fungi





Similar content being viewed by others
References
Azerad R (1999) Adv Biochem Eng Biotechnol 63:169–218
Pupo MT, Borges KB, Borges WS, Bonato PS (2008) Fungal biotransformations: a powerful tool in drug metabolism studies. In: Saikai R, Bezbaruah RL, Bora TCh (eds) Microbial biotechnology. New India Publishing Agency, New Delhi, pp 47–66
Borges KB, Borges WS, Durán-Patrón R, Pupo MT, Bonato PS, Collado IG (2009) Tetrahedron Asymmetr 20:385–397
Fura A, Shu Y-Z, Zhu M, Hanson RL, Roongta V, Humphreys WG (2004) J Med Chem 47:4339–4351
Smith RV, Rosazza JP (1983) J Nat Prod 46:79–91
Abourashed EA, Clark AM, Hufford CD (1999) Curr Med Chem 6:359–364
Lisowska K, Szemraj J, Rozalska S, Dlugonski J (2006) FEMS Microbiol Lett 261:175–180
Zhang D, Zhang H, Aranibar N, Hanson R, Huang Y, Cheng PT, Shung W, Bonacorsi S, Mingshe Z, Swaminathan A, Humphreys WG (2006) Drug Metab Dispos 34:267–280
Lisowska K, Dlugonski J, Freeman JP, Cerniglia CE (2006) Chemosphere 64:1499–1506
Borges KB, Borges WS, Pupo MT, Bonato PS (2007) Appl Microbiol Biotechnol 77:669–674
Borges KB, Borges WS, Pupo MT, Bonato PS (2008) J Pharm Biomed Anal 46:945–952
Borges KB, Pupo MT, Bonato PS (2009) Electrophoresis 30:3910–3917
Borges WS, Borges KB, Bonato PS, Said S, Pupo MT (2009) Curr Org Chem 13:1137–1163
Barth T, Pupo MT, Borges KB, Okano LT, Bonato PS (2010) Electrophoresis 31:1521–1528
Wilson D (1995) Oikos 73:274–276
Schulz B, Boyle C (2005) Mycol Res 109:661–686
Nicoll-Griffith D, Scartozzi M, Chiem N (1993) J Chromatogr A 653:253–259
Oliveira ARM, Cesarino EJ, Bonato OS (2005) J Chromatogr B 818:285–291
Augusti DV, Augusti R (2005) Tetrahedron Asymmetr 16:1881–1885
Adams SS, Bresloff P, Mason CG (1976) J Pharm Pharmacol 28:256–257
Hutt AJ, Caldwell J (1983) J Pharm Pharmacol 35:693–704
Paliwal JK, Smith DE, Cox SR, Berardi RR, Dunn-Kucharski VA, Elta GH (1993) J Pharmacokinet Biopharm 21:145–161
Davies NM (1998) Clin Pharmacokinet 34:101–154
Hamman MA, Thompson GA, Hall SD (1997) Biochem Pharmacol 54:33–41
Guimarães DO, Borges WS, Kawano CY, Ribeiro PH, Goldman GH, Nomizo A, Thiemann OH, Oliva G, Lopes NP, Pupo MT (2008) FEMS Immunol Med Microbiol 52:134–144
Momesso LS, Kawano CY, Ribeiro PH, Nomizo A, Goldman GH, Pupo MT (2008) Quim Nova 31:1680–1685
Gallo MBC, Chagas FO, Almeida MO, Macedo CC, Cavalcanti BC, Barros FWA, Moraes MO, Costa-Lotuffo LV, Pessoa CO, Bastos JK, Pupo MT (2009) J Basic Microbiol 49:142–151
de Oliveira ARM, de Santanta FJM, Bonato PS (2005) Anal Chim Acta 538:25–34
Tan SC, Jackson SHD, Swift CG, Hutt AJ (1997) J Chromatogr B 701:53–63
Acknowledgements
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support and for granting research fellowships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borges, K.B., de Oliveira, A.R.M., Barth, T. et al. LC–MS–MS determination of ibuprofen, 2-hydroxyibuprofen enantiomers, and carboxyibuprofen stereoisomers for application in biotransformation studies employing endophytic fungi. Anal Bioanal Chem 399, 915–925 (2011). https://doi.org/10.1007/s00216-010-4329-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4329-9