Abstract
Since 2004, cannabis has been prohibited by the World Anti-Doping Agency for all sports competitions. In the years since then, about half of all positive doping cases in Switzerland have been related to cannabis consumption. In doping urine analysis, the target analyte is 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THC-COOH), the cutoff being 15 ng/mL. However, the wide urinary detection window of the long-term metabolite of Δ9-tetrahydrocannabinol (THC) does not allow a conclusion to be drawn regarding the time of consumption or the impact on the physical performance. The purpose of the present study on light cannabis smokers was to evaluate target analytes with shorter urinary excretion times. Twelve male volunteers smoked a cannabis cigarette standardized to 70 mg THC per cigarette. Plasma and urine were collected up to 8 h and 11 days, respectively. Total THC, 11-hydroxy-Δ9-tetrahydrocannabinol (THC-OH), and THC-COOH were determined after hydrolysis followed by solid-phase extraction and gas chromatography/mass spectrometry. The limits of quantitation were 0.1–1.0 ng/mL. Eight puffs delivered a mean THC dose of 45 mg. Plasma levels of total THC, THC-OH, and THC-COOH were measured in the ranges 0.2–59.1, 0.1–3.9, and 0.4–16.4 ng/mL, respectively. Peak concentrations were observed at 5, 5–20, and 20–180 min. Urine levels were measured in the ranges 0.1–1.3, 0.1–14.4, and 0.5–38.2 ng/mL, peaking at 2, 2, and 6–24 h, respectively. The times of the last detectable levels were 2–8, 6–96, and 48–120 h. Besides high to very high THC-COOH levels (245 ± 1,111 ng/mL), THC (3 ± 8 ng/mL) and THC-OH (51 ± 246 ng/mL) were found in 65 and 98% of cannabis-positive athletes’ urine samples, respectively. In conclusion, in addition to THC-COOH, the pharmacologically active THC and THC-OH should be used as target analytes for doping urine analysis. In the case of light cannabis use, this may allow the estimation of more recent consumption, probably influencing performance during competitions. However, it is not possible to discriminate the intention of cannabis use, i.e., for recreational or doping purposes. Additionally, pharmacokinetic data of female volunteers are needed to interpret cannabis-positive doping cases of female athletes.

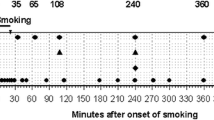
Urine concentration ranges of delta-9-tetrahydrocannabinol (THC) and its metabolites 11-hydroxy-delta-9-tetrahydrocannabinol (THC-OH) and 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THC-COOH) in athletes tested cannabispositive (15 ng/mL THC-COOH or more; N=81)






Similar content being viewed by others
References
Thevis M, Kuuranne T, Geyer H, Schänzer W (2009) Drug Test Anal 1:4–13
Antidoping Agency Switzerland (2009) List of prohibited substances and methods (doping list). Antidoping Agency Switzerland, Berne
Saugy M, Avois L, Saudan C, Robinson N, Giroud C, Mangin P, Dvorak J (2006) Br J Sports Med 40(Suppl 1):i13–i15
Lorente FO, Peretti-Watel P, Grelot L (2005) Addict Behav 30:1382–1391
Wall ME, Perez-Reyes M (1981) J Clin Pharmacol 21:178S–189S
Fraser AD, Worth D (2004) Forensic Sci Int 143:147–152
Mareck U, Fusshöller G, Geyer H, Haenelt N, Thevis M, Kamber M, Brenneisen R, Schänzer W (2006) In: Schänzer W, Geyer H, Gotzmann A, Mareck U (eds) Recent advances in doping analysis (14). Sport und Buch Strauss, Cologne, pp 101–109
Kamber M, Hintz O (2005) Annual report 2005. Antidoping Switzerland, Berne
Musshoff F, Madea B (2006) Ther Drug Monit 28:155–163
Baselt RC, Cravey RH (1995) Disposition of toxic drugs and chemicals in man, 4th edn. Chemical Toxicology Institute, Foster City
Perez-Reyes M, Lipton MA, Timmons MC, Wall ME, Brine DR, Davis KH (1973) Clin Pharmacol Ther 14:48–55
Lowe RH, Abraham TT, Darwin WD, Herning R, Cadet JL, Huestis MA (2009) Drug Alcohol Depend 105:24–32
Perez-Reyes M, Timmons MC, Lipton MA, Davis KH, Wall ME (1972) Science 177:633–635
Christensen HD, Freudenthal RI, Gidley JT, Rosenfeld R, Boegli G, Testino L, Brine DR, Pitt CG, Wall ME (1971) Science 172:165–167
Iversen L (2000) The science of marijuana. Oxford University Press, Oxford
Cone EJ, Huestis MA (1993) Ther Drug Monit 15:527–532
Abraham TT, Lowe RH, Pirnay SO, Darwin WD, Huestis MA (2007) J Anal Toxicol 31:477–485
Huestis M (1999) In: Nahas GG, Sutin K, Harvey D, Agurell S (eds) Marihuana and medicine. Humana, Totowa, pp 105–116
McGilveray IJ (2005) Pain Res Manag 10:15A–22A
Manno JE, Manno BR, Kemp PM, Alford DD, Abukhalaf IK, McWilliams ME, Hagaman FN, Fitzgerald MJ (2001) J Anal Toxicol 25:538–549
Skopp G, Potsch L (2008) J Anal Toxicol 32:160–164
Leighty EG, Fentiman AF Jr, Foltz RL (1976) Res Commun Chem Pathol Pharmacol 14:13–28
Johansson E, Halldin MM, Agurell S, Hollister LE, Gillespie HK (1989) Eur J Clin Pharmacol 37:273–277
Haggerty GC, Deskin R, Kurtz PJ, Fentiman AF, Leighty EG (1986) Toxicol Appl Pharmacol 84:599–606
Niedbala RS, Kardos KW, Fritch DF, Kardos S, Fries T, Waga J, Robb J, Cone EJ (2001) J Anal Toxicol 25:289–303
Toennes SW, Kauert GF, Steinmeyer S, Moeller MR (2005) Forensic Sci Int 152:149–155
Fant RV, Heishman SJ, Bunker EB, Pickworth WB (1998) Pharmacol Biochem Behav 60:777–784
Heishman SJ, Stitzer ML, Bigelow GE (1988) Pharmacol Biochem Behav 31:649–655
Brenneisen R, ElSohly MA (1988) J Forensic Sci 33:1385–1404
Feng S, ElSohly MA, Salamone S, Salem MY (2000) J Anal Toxicol 24:395–402
International Conference on Harmonization (1996) Validation of analytical methods: methodology ICH Q2 B
Peters FT, Hartung M, Herbold M, Schmitt G, Daldrup T, Musshoff F (2004) Toxichem Krimtech 71:146–154
Peters FT, Drummer OH, Musshoff F (2007) Forensic Sci Int 165:216–224
Davies KH, McDaniel IA, Caddel LW, Moody PL (1984) In: Agurell S, Dewey WL, Willette RE (eds) The cannabinoids: chemical, pharmacologic, and therapeutic aspects. Academic, New York, pp 97–109
Perez-Reyes M (1990) NIDA Res Monogr 99:42–62
Huestis MA, Henningfield JE, Cone EJ (1992) J Anal Toxicol 16:276–282
Kauert GF, Ramaekers JG, Schneider E, Moeller MR, Toennes SW (2007) J Anal Toxicol 31:288–293
Hunt CA, Jones RT (1980) J Pharmacol Exp Ther 215:35–44
Agurell S, Halldin M, Hollister L (1990) In: Watson R (ed) Biochemistry and physiology of substance abuse. CRC, Boca Raton, pp 138–172
McBurney LJ, Bobbie BA, Sepp LA (1986) J Anal Toxicol 10:56–64
Hollister L, Gillespie H, Ohlsson A, Lindgren J, Wahlen A, Agurell S (1981) J Clin Pharmacol 21:171S–177S
Huestis MA, Mitchell JM, Cone EJ (1996) J Anal Toxicol 20:441–452
Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M (1983) Clin Pharmacol Ther 34:352–363
Acknowledgements
Special thanks go to the staff of the CIU and Arno Hazekamp, University of Leiden, for assisting in the supply of the test material (Bedrobinol®). The study was supported by Antidoping Switzerland, Berne, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Plasma and urine profiles of Δ9-tetrahydrocannabinol and its metabolites 11-hydroxy-Δ9-tetrahydrocannabinol and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol after cannabis smoking by male volunteers to estimate recent consumption by athletes (PDF 2053 kb)
Rights and permissions
About this article
Cite this article
Brenneisen, R., Meyer, P., Chtioui, H. et al. Plasma and urine profiles of Δ9-tetrahydrocannabinol and its metabolites 11-hydroxy-Δ9-tetrahydrocannabinol and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol after cannabis smoking by male volunteers to estimate recent consumption by athletes. Anal Bioanal Chem 396, 2493–2502 (2010). https://doi.org/10.1007/s00216-009-3431-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3431-3