Abstract
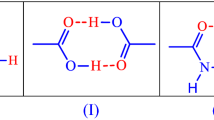
Raman spectroscopy, X-ray powder diffraction/X-ray crystallography and differential scanning calorimetry have been used to study the phenomenon of co-crystal formation in stoichiometric mixtures of salicylic acid with benzamide. Raman spectroscopy was particularly useful for the characterization of the products and was used to determine the nature of the interactions in the co-crystals. It was observed that little change in the vibrational modes associated with the phenyl groups of the respective reactants took place upon co-crystal formation, but changes in intensities of the vibrational modes associated with the amide and the carboxylic acid groups were observed upon co-crystal formation. Several new vibrational bands were identified in the co-crystal which were not manifested in the physical mixture of both components and could be used as diagnostic features of co-crystal formation.














Similar content being viewed by others
References
Aakeroy CB, Salmon DJ (2005) Cryst Eng Commun 7:439–448
Morissette SL, Almarsson O, Peterson ML, Remenar JF, Read MJ, Lemmo AV, Ellis S, Cima MJ, Gardner CR (2004) Adv Drug Deliv Rev 56:275–300
Peterson ML, Hickey MB, Zaworotko MJ, Almarsson O (2006) J Pharm Pharmaceut Sci 9:317–326
Jones W (1997) Organic molecular solids: properties and applications. CRC, New York
Atwood JL, Davies JED, MacNicol DD, Vogtle F (1996) Comprehensive supramolecular chemistry, vol 9. Pergamon, Oxford
Bond AD, Jones W (2002) Supramolecular organization and materials design. Cambridge University Press, Cambridge
Desiraju GR (1989) Crystal engineering: the design of organic solids. Elsevier, New York
Banerjee R, Bhatt PM, Ravindra NV, Desiraju GM (2005) Cryst Growth Des 6:2299–2309
Chiarella RA, Davey RJ, Peterson ML (2007) Cryst Growth Des 7:1223–1226
Shan N, Zaworotko MJ (2008) Drug Discov Today 13:440–446
Almarsson O, Zaworotko MJ (2004) Chem Commun 17:1889–1896
Shattock TR, Arora KK, Vishweshwar P, Zaworotko MJ (2008) Cryst Growth Des 8:4533–4545
Hickey MB, Peterson ML, Scoppettuolo LA, Morrisette SL, Vetter A, Guzman H, Remenar JF, Zhang Z, Tawa MD, Haley S, Zaworotko MJ, Almarssono O (2007) Eur J Pharm Biopharm 67:112–119
Chadwick K, Davey R, Cross W (2007) Cryst Eng Commun 9:732–734
Palmer DS, Llinas A, Morao I, Day GM, Goodman JM, Glen RC, Mitchell JBO (2008) Mol Pharm 5:266–279
Seefeldt K, Miller J, Alvarez-Nunez F, Rodrıguez-Hornedo N (2007) J Pharm Sci 96:1147–1158
Zhang GGZ, Henry RF, Borchardt TB, Lou XC (2007) J Pharm Sci 96:990–995
Takata N, Shiraki K, Takano R, Hayashi Y, Terada K (2008) Cryst Growth Des 8:3032–3037
Bis JA, Vishweshwar P, Middleton RA, Zaworotko MJ (2006) Cryst Growth Des 6:1048–1053
Fleishman SG, Kuduva SS, McMahon JA, Moulton B, Rosa D, Bailey W, Rodrıguez-Hornedo N, Zaworotko MJ (2003) Cryst Growth Des 3:909–919
Allen FH, Motherwell WDS, Raithby PR, Shields GP, Taylor R (1999) New J Chem 23:25–34
Arkema S, Bats JW, Weyenberg AM, Feil D (1972) Acta Crystallogr B 28:1646–1648
Leiserowitz L, Nader F (1977) Acta Crystallogr B 33:2719–2733
Reddy LS, Nangia A, Lynch VM (2004) Cryst Growth Des 4:89–94
Aakeroy CB, Beatty AM, Helfrich BA, Nieuwenhuyzen M (2003) Cryst Growth Des 3:159–165
Vishweshwar P, Nangia A, Lynch VM (2002) J Org Chem 67:556–565
Vishweshwar P, Nangia A, Lynch VM (2003) Cryst Growth Des 3:783–790
Allen FA (2002) Acta Crystallogr Sect B 58:380
Aakeroy CB, Beatty AM, Nieuwenhuyzen M, Zou M (2000) Tetrahedron 56:6693–6699
Childs SL, Stahly GP, Park A (2007) Mol Pharm 4:323–338
Blake CCF, Small RWH (1972) Acta Crystallogr B 28:2201–2206
Boczar M, Boda L, Wojcik MJ (2006) Spectrochim Acta Part A 64:757–760
Volovsek V, Colombo L, Furic K (1983) J Raman Spect 14:347–352
Humbret B, Alnot M, Quiles F (1998) Spectrochim Acta Part A 54:465–476
Kniseley RN, Fassel VA, Farquhar EL, Gray LS (1962) Spectrochim Acta 18:1217–1229
Smith BC (1998) Infrared spectral interpretation: a systematic approach. CRC, Boca Raton
Additional Information Available
X-ray crystallographic information for the crystal structures (PDF); this material is available free of charge via the Internet at http://pubs.acs.org. Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. CCDC-756492. Copies of available material can be obtained, free of charge, on application to the Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: + 44 (0) 1223-336033 or e mail: teched@chemcrys.cam.ac.uk).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 450 kb)
Rights and permissions
About this article
Cite this article
Elbagerma, M.A., Edwards, H.G.M., Munshi, T. et al. Identification of a new co-crystal of salicylic acid and benzamide of pharmaceutical relevance. Anal Bioanal Chem 397, 137–146 (2010). https://doi.org/10.1007/s00216-009-3375-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3375-7