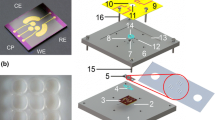
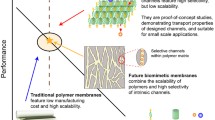
Abstract
Biological membranes constitute the set of membranes defining boundaries and organelles in living cells—the structural and functional building blocks of all known living organisms. The integrity of the cell depends on its ability to separate inside from outside and yet at the same time allow massive transport of matter in and out the cell. Nature has elegantly met this challenge by developing membranes in the form of lipid bilayers in which specialized and highly efficient transport proteins are incorporated. This raises the question: is it possible to mimic biological membranes and create membrane-based sensor and/or separation devices? In the development of biomimetic sensor/separation technology, both channels (ion and water channels) and carriers (transporters) are important. Generally, each class of transport proteins conducts specific molecular species in and out of the cell while preventing the passage of others, a property critical for the overall conservation of the cells internal pH and salt concentration. Both ion and water channels are highly efficient membrane pore proteins capable of transporting solutes at very high rates, up to 109 molecules per second. Carrier proteins generally have a lower turnover but are capable of transport against gradients. For both classes of proteins, their unique flux-properties make them interesting as candidates in biomimetic sensor/separation devices. An ideal sensor/separation device requires the supporting biomimetic matrix to be virtually impermeable to anything but the solute in question. In practice, however, a biomimetic support matrix will generally have finite permeabilities to water, electrolytes, and non-electrolytes. The feasibility of a biomimetic device thus depends on the relative transport contribution from both protein and biomimetic support matrix. Also the stability of the incorporated protein must be addressed and the protein-biomimetic matrix must be encapsulated in order to protect it and make it sufficiently stable in a final application. Here I will review and discuss these challenges and how they are met in some current developments of biomimetic sensor/separation devices.









Similar content being viewed by others
References
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Molecular biology of the cell, 4th edn. Garland Science, New York
Yeagle PL (ed) (2005) The structure of biological membranes. CRC Press, Boca Raton
Gennis RB (1989) Biomembranes. Molecular structure and function. Springer, New York
Andersen OS (1978) Permeability properties of unmodified lipid bilayer membranes. Membr Transp Biol 1:369–446
Hille B (1992) Ionic channels of excitable membranes, 2nd edn. Sinauer Associates, Sunderland
Jensen MO, Mouritsen OG (2006) Single-channel water permeabilities of Escherichia coli aquaporins AqpZ and GlpF. Biophys J 90:2270–2284
Stock D, Gibbons C, Arechaga I, Leslie AG, Walker JE (2000) The rotary mechanism of ATP synthase. Curr Opin Struct Biol 10:672–679
Dowhan W (1997) Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu Rev Biochem 66:199–232
Dopico AM, Tigyi GJ (2007) A glance at the structural and functional diversity of membrane lipids. Methods Mol Biol 400:1–13
Pomorski T, Menon AK (2006) Lipid flippases and their biological functions. Cell Mol Life Sci 63:2908–2921
Sackmann E (1984) Physical basis of trigger processes and membrane structures. In: Chapman D (ed) Biological membranes. Academic Press Inc., Ltd., London, pp 105–143
Vind-Kezunovic D, Nielsen CH, Wojewodzka U, Gniadecki R (2008) Line tension at lipid phase boundaries regulates formation of membrane vesicles in living cells. Biochim Biophys Acta 1778:2480–2486
Lundbæk JA (2006) Regulation of membrane protein function by lipid bilayer elasticity - a single molecule technology to measure the bilayer properties experienced by an embedded protein. J Phys Condens Matter 18:S1305–S1344
Nielsen CH (2009) Lipid-protein interactions in biomembranes. In: Bohr HG (ed) Handbook of biophysics. Wiley, Berlin, pp 329–358
Niggli V (2005) Regulation of protein activities by phosphoinositide phosphates. Annu Rev Cell Dev Biol 21:57–79
Andersen OS, Koeppe RE 2nd (2007) Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct 36:107–130
Nielsen C, Goulian M, Andersen OS (1998) Energetics of inclusion-induced bilayer deformations. Biophys J 74:1966–1983
Mueller P, Rudin DO, Tien HT, Wescott WC (1962) Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 194:979–980
Montal M, Mueller P (1972) Formation of biomolecular membranes from lipid monolayers and a study of their electrical properties. Proc Nat Acad Sci USA 69:3561–3566
White SH (1978) Formation of “solvent-free” black lipid bilayer membranes from glyceryl monooleate dispersed in squalene. Biophys J 23:337–347
Cruz A, Perez-Gil J (2007) Langmuir films to determine lateral surface pressure on lipid segregation. Methods Mol Biol 400:439–457
Lin WC, Blanchette CD, Ratto TV, Longo ML (2007) Lipid domains in supported lipid bilayer for atomic force microscopy. Methods Mol Biol 400:503–513
Watts TH, Brian AA, Kappler JW, Marrack P, McConnell HM (1984) Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc Natl Acad Sci U S A 81:7564–7568
McConnell HM, Watts TH, Weis RM, Brian AA (1986) Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim Biophys Acta 864:95–106
Lin WC, Blanchette CD, Ratto TV, Longo ML (2006) Lipid asymmetry in DLPC/DSPC-supported lipid bilayers: a combined AFM and fluorescence microscopy study. Biophys J 90:228–237
Elie-Caille C, Fliniaux O, Pantigny J, Maziere JC, Bourdillon C (2005) Self-assembly of solid-supported membranes using a triggered fusion of phospholipid-enriched proteoliposomes prepared from the inner mitochondrial membrane. Langmuir 21:4661–4668
Ti Tien H (1974) Bilayer lipid membranes (BML) theory and practice. Marcel Dekker Inc, New York
Tien HT, Ottova-Leitmannova A (eds) (2003) Planar lipid bilayers (BLMs) and their applications. Elsevier, Amsterdam
Ottova A, Tien HT (2002) The 40th anniversary of bilayer lipid membrane research. Bioelectrochemistry 56:171–173
Mayer M, Kriebel JK, Tosteson MT, Whitesides GM (2003) Microfabricated Teflon membranes for low-noise recordings of ion channels in planar lipid bilayers. Biophys J 85:2684–2695
Suzuki H, Tabata KV, Noji H, Takeuchi S (2006) Highly reproducible method of planar lipid bilayer reconstitution in polymethyl methacrylate microfluidic chip. Langmuir 22:1937–1942
Malmstadt N, Nash MA, Purnell RF, Schmidt JJ (2006) Automated formation of lipid-bilayer membranes in a microfluidic device. Nano Lett 6:1961–1965
Zagnoni M, Sandison ME, Morgan H (2009) Microfluidic array platform for simultaneous lipid bilayer membrane formation. Biosens Bioelectron 24:1235–1240
Sandison ME, Zagnoni M, Abu-Hantash M, Morgan H (2007) Micromachined glass apertures for artificial lipid bilayer formation in a microfluidic system. J Micromech Microeng 17:S189–S196
Hansen JS, Perry ME, Vogel J, Vissing T, Geschke O, Emneus J, Nielsen CH (2009) Development of an automation technique for the establishment of functional lipid bilayer arrays. J Micromech Microeng 19:025014
Vogel J, Perry ME, Hansen JS, Bollinger P-Y, Nielsen CH, Geschke O (2009) Support structure for biomimetic applications. J Micromech Microeng 19:025026
van de Vossenberg JL, Driessen AJ, Konings WN (1998) The essence of being extremophilic: the role of the unique archaeal membrane lipids. Extremophiles 2:163–170
Schleper C, Puehler G, Holz I, Gambacorta A, Janekovic D, Santarius U, Klenk HP, Zillig W (1995) Picrophilus gen. nov., fam. nov.: a novel aerobic, heterotrophic, thermoacidophilic genus and family comprising archaea capable of growth around pH 0. J Bacteriol 177:7050–7059
van de Vossenberg J, Driessen AJ, Zillig W, Konings WN (1998) Bioenergetics and cytoplasmic membrane stability of the extremely acidophilic, thermophilic archaeon Picrophilus oshimae. Extremophiles 2:67–74
De Rosa M, Gambacorta A (1988) The lipids of archaebacteria. Prog Lipid Res 27:153–175
Dannenmuller O, Arakawa K, Eguchi T, Kakinuma K, Blanc S, Albrecht AM, Schmutz M, Nakatani Y, Ourisson G (2000) Membrane properties of archaeal macrocyclic diether phospholipids. Chemistry 6:645–654
Chang EL (1994) Unusual thermal stability of liposomes made from bipolar tetraether lipids. Biochem Biophys Res Commun 202:673–679
Holland DP, Struts AV, Brown MF, Thompson DH (2008) Bolalipid membrane structure revealed by solid-state 2H NMR spectroscopy. J Am Chem Soc 130:4584–4585
Wiess-Wichert C, Smetazko M, Valina-Saba M, Schalkhammer T (1997) A new analytical device based on gated ion channels: a peptide-channel biosensor. J Biol Screening 2:11–18
Cornell BA, Braach-Maksvytis VL, King LG, Osman PD, Raguse B, Wieczorek L, Pace RJ (1997) A biosensor that uses ion-channel switches. Nature 387:580–583
Febo-Ayala W, Morera-Felix SL, Hrycyna CA, Thompson DH (2006) Functional reconstitution of the integral membrane enzyme, isoprenylcysteine carboxyl methyltransferase, in synthetic bolalipid membrane vesicles. Biochemistry 45:14683–14694
Svenson S, Thompson DH (1998) Facile and efficient synthesis of bolaamphiphilic tetraether phosphocholines. J Org Chem 63:7180–7182
Meier W, Nardin C, Winterhalter M (2000) Reconstitution of channel proteins in (polymerized) ABA triblock copolymer membranes. Angew Chem Int Ed Engl 39:4599–4602
Ho D, Chu B, Lee H, Montemagno C (2004) Protein-driven transduction across polymeric biomembranes. Nanotechnology 15:1084–1094
Kumar M, Grzelakowski M, Zilles J, Clark M, Meier W (2007) Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. Proc Natl Acad Sci U S A 104:20719–20724
Reimhult E, Kumar K (2008) Membrane biosensor platforms using nano- and microporous supports. Trends Biotechnol 26:82–89
Separovic F, Cornell BA (2007) Gated ion channel-based biosensor device. In: Chung SH, Andersen OS, Krishnamurthy V (eds) Biological membrane ion channels. Springer, New York, pp 595–622
Eray M, Dogan NS, Reiken SR, Sutisna H, Van Wie BJ, Koch AR, Moffett DF, Silber M, Davis WC (1995) A highly stable and selective biosensor using modified nicotinic acetylcholine receptor (nAChR). Biosystems 35:183–188
Zhang L, Granick S (2006) Dynamical heterogeneity in supported lipid bilayers. MRS Bull 31:527–531
Tanaka M (2006) Polymer-supported membranes: physical models of cell surfaces. MRS Bull 31:513–520
Parikh AN, Groves JT (2006) Materials science of supported lipid membranes. MRS Bull 31:507–512
Sackmann E, Tanaka M (2000) Supported membranes on soft polymer cushions: fabrication, characterization and applications. Trends Biotechnol 18:58–64
Sackmann E (1996) Supported membranes: scientific and practical applications. Science 271:43–48
Rossi C, Chopineau J (2007) Biomimetic tethered lipid membranes designed for membrane-protein interaction studies. Eur Biophys J 36:955–965
Koper I (2007) Insulating tethered bilayer lipid membranes to study membrane proteins. Mol Biosyst 3:651–657
Gagner J, Johnson H, Watkins E, Li Q, Terrones M, Majewski J (2006) Carbon nanotube supported single phospholipid bilayer. Langmuir 22:10909–10911
Castellana ET, Cremer PS (2006) Solid supported lipid bilayers: from biophysical studies to sensor design. Surf Sci Rep 61:429–444
Murray DH, Tamm LK, Kiessling V (2009) Supported double membranes. J Struct Biol (in press)
Löfås S, Johnsson BJ (1990) A novel hydrogel matrix on gold surfaces in surface plasmon resonance sensors for fast and efficient covalent immobilization of ligands. Chem Soc Chem Commun 21:1526–1528
Dong Y, Scott Phillips K, Chen Q (2006) Immunosensing of Staphylococcus enterotoxin B (SEB) in milk with PDMS microfluidic systems using reinforced supported bilayer membranes. Lab Chip 6:675–681
Steltze M (1993) On the application of supported bilayers as receptive layers for biosensors with electrical detection. J Phys Chem 97:2974–2981
Becucci L, Leon RR, Moncelli MR, Rovero P, Guidelli R (2006) Electrochemical investigation of melittin reconstituted into a mercury-supported lipid bilayer. Langmuir 22:6644–6650
Becucci L, Moncelli MR, Naumann R, Guidelli R (2005) Potassium ion transport by valinomycin across a Hg-supported lipid bilayer. J Am Chem Soc 127:13316–13323
Becucci L, Moncelli MR, Guidelli R (2006) Impedance spectroscopy of OmpF porin reconstituted into a mercury-supported lipid bilayer. Langmuir 22:1341–1346
Ho D, Chu B, Lee H, Montemagno C (2004) Protein-driven energy transduction across polymeric biomembranes. Nanotechnology 15:1084–1094
Holden MA, Needham D, Bayley H (2007) Functional bionetworks from nanoliter water droplets. J Am Chem Soc 129:8650–8655
Funakoshi K, Suzuki H, Takeuchi S (2006) Lipid bilayer formation by contacting monolayers in a microfluidic device for membrane protein analysis. Anal Chem 78:8169–8174
Zagnoni M, Sandison ME, Marius P, Morgan H (2009) Bilayer lipid membranes from falling droplets. Anal Bioanal Chem 393:1601–1605
Andersen OS, Green WN, Urban BW (1986) Ion conduction through sodium channels in planar lipid bilayers. Ion Channel Reconstitution 385-404
Benz R, Janko K (1976) Voltage-dependent capacitance relaxation of lipid bilayer membranes. Effects of membrane composition. Biochim Biophys Acta 455:721–738
Jeon TJ, Malmstadt N, Schmidt JJ (2006) Hydrogel-encapsulated lipid membranes. J Am Chem Soc 128:42–43
Jeon TJ, Poulos JL, Schmidt J (2008) Storable and transportable lipid bilayer membrane precursor. Biophys J Abstracts 337a
Shim JW, Gu LQ (2007) Stochastic sensing on a modular chip containing a single-ion channel. Anal Chem 79:2207–2213
Schuster B, Sleytr UB, Diederich A, Bahr G, Winterhalter M (1999) Probing the stability of S-layer-supported planar lipid membranes. Eur Biophys J 28:583–590
Schuster B, Sleytr UB (2002) The effect of hydrostatic pressure on S-layer-supported lipid membranes. Biochim Biophys Acta 1563:29–34
Sleytr UB, Egelseer EM, Ilk N, Pum D, Schuster B (2007) S-Layers as a basic building block in a molecular construction kit. FEBS J 274:323–334
Kang XF, Cheley S, Rice-Ficht AC, Bayley H (2007) A storable encapsulated bilayer chip containing a single protein nanopore. J Am Chem Soc 129:4701–4705
Kaufman Y, Berman A, Freger V (2008) The use of NF membranes as substrates for supported phospholipid bilayers. ICOM 2008 Poster Session Proceedings, International Congress on Membranes and Membrane Processes, Honolulu, Hawaii, July 12–18, p 560
Ndoni S, Vigild ME, Berg RH (2003) Nanoporous materials with spherical and gyroid cavities created by quantitative etching of polydimethylsiloxane in polystyrene-polydimethylsiloxane block copolymers. J Am Chem Soc 125:13366–13367
Hansen MS, Vigild ME, Berg RH, Ndoni S (2004) Nanoporous crosslinked polyisoprene from polyisoprene - Polydimethylsiloxane block copolymer. Polymer Bulletin 51:403–409
Lodge TP (2003) Block copolymers: past successes and future challenges. Macromol Chem Phys 204:265–273
Park C, Yoon J, Thomas EL (2003) Enabling nanotechnology with self assembled block copolymer patterns. Polymer 44:6725–6760
Lian J, Ding S, Cai J, Zhang D, Xu Z, Wang X (2009) Improving aquaporin Z expression in Escherichia coli by fusion partners and subsequent condition optimization. Appl Microbiol Biotechnol 82:463–470
Kiefer H, Krieger J, Olszewski JD, Von Heijne G, Prestwich GD, Breer H (1996) Expression of an olfactory receptor in Escherichia coli: purification, reconstitution, and ligand binding. Biochemistry 35:16077–16084
Tate CG (2001) Overexpression of mammalian integral membrane proteins for structural studies. FEBS Lett 504:94–98
Chen GQ, Gouaux JE (1996) Overexpression of bacterio-opsin in Escherichia coli as a water-soluble fusion to maltose binding protein: efficient regeneration of the fusion protein and selective cleavage with trypsin. Protein Sci 5:456–467
LaVallie ER, DiBlasio EA, Kovacic S, Grant KL, Schendel PF, McCoy JM (1993) A thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Biotechnology (NY) 11:187–193
Nygren PA, Stahl S, Uhlen M (1994) Engineering proteins to facilitate bioprocessing. Trends Biotechnol 12:184–188
Pryor KD, Leiting B (1997) High-level expression of soluble protein in Escherichia coli using a His6-tag and maltose-binding-protein double-affinity fusion system. Protein Exp Purif 10:309–319
Samuelsson E, Moks T, Nilsson B, Uhlen M (1994) Enhanced in vitro refolding of insulin-like growth factor I using a solubilizing fusion partner. Biochemistry 33:4207–4211
Power RF, Conneely OM, McDonnell DP, Clark JH, Butt TR, Schrader WT, O’Malley BW (1990) High level expression of a truncated chicken progesterone receptor in Escherichia coli. J Biol Chem 265:1419–1424
Woolley GA, Wallace BA (1992) Model ion channels: gramicidin and alamethicin. J Membrane Biol 129:109–136
Becucci L, Carbone MV, Biagiotti T, D’Amico M, Olivotto M, Guidelli R (2008) Incorporation of the HERG potassium channel in a mercury supported lipid bilayer. J Phys Chem B 112:1315–1319
Racker E (1972) Reconstitution of cytochrome oxidase vesicles and conferral of sensitivity to energy transfer inhibitors. J Membr Biol 10:221–235
Racker E (1973) A new procedure for the reconstitution of biologically active phospholipid vesicles. Biochem Biophys Res Commun 55:224–230
Hengen P (1995) Purification of His-Tag fusion proteins from Escherichia coli. Trends Biochem Sci 20:285–286
Deniaud A, Rossi C, Berquand A, Homand J, Campagna S, Knoll W, Brenner C, Chopineau J (2007) Voltage-dependent anion channel transports calcium ions through biomimetic membranes. Langmuir 23:3898–3905
Sheibani N (1999) Prokaryotic gene fusion expression systems and their use in structural and functional studies of proteins. Prep Biochem Biotechnol 29:77–90
Terpe K (2003) Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 60:523–533
Nardin C, Thoeni S, Widmer J, Winterhalter M, Meier W (2000) Chem Commun (Camb) 15:1433
Choi HJ, Montemagno CD (2005) Artificial organelle: ATP synthesis from cellular mimetic polymersomes. Nano Lett 5:2538–2542
Choi HJ, Montemagno CD (2005) Nanotechnology 16:1589
Woodbury DJ, Miller C (1990) Nystatin-induced liposome fusion. A versatile approach to ion channel reconstitution into planar bilayers. Biophys J 58:833–839
Cass A, Finkelstein A, Krespi V (1970) The ion permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol 56:100–124
Moreno-Bello M, Bonilla-Marin M, Gonzalez-Beltran C (1988) Distribution of pore sizes in black lipid membranes treated with nystatin. Biochim Biophys Acta 944:97–100
Woodbury DJ (1999) Nystatin/ergosterol method for reconstituting ion channels into planar lipid bilayers. Methods Enzymol 294:319–339
Kalb E, Frey S, Tamm LK (1992) Formation of supported planar bilayers by fusion of vesicles to supported phospholipid monolayers. Biochim Biophys Acta 1103:307–316
Crane JM, Tamm LK (2007) Fluorescence microscopy to study domains in supported lipid bilayers. Methods Mol Biol 400:481–488
Malinin VS, Frederik P, Lentz BR (2002) Osmotic and curvature stress affect PEG-induced fusion of lipid vesicles but not mixing of their lipids. Biophys J 82:2090–2100
Lentz BR (1994) Polymer-induced membrane fusion: potential mechanism and relation to cell fusion events. Chem Phys Lipids 73:91–106
Simberg D, Weisman S, Talmon Y, Barenholz Y (2004) DOTAP (and other cationic lipids): chemistry, biophysics, and transfection. Crit Rev Ther Drug Carrier Syst 21:257–317
Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE (1998) SNAREpins: minimal machinery for membrane fusion. Cell 92:759–772
Wang T, Smith EA, Chapman ER, Weisshaar JC (2009) Lipid mixing and content release in single-vesicle, SNARE-driven fusion assay with 1–5 ms resolution. Biophys J 96:4122–4131
Van Gelder P, Dumas F, Rosenbusch JP, Winterhalter M (2000) Oriented channels reveal asymmetric energy barriers for sugar translocation through maltoporin of Escherichia coli. Eur J Biochem 267:79–84
Dargent B, Hofmann W, Pattus F, Rosenbusch JP (1986) The selectivity filter of voltage-dependent channels formed by phosphoporin (PhoE protein) from E. coli. EMBO J 5:773–778
Van Gelder P, Saint N, van Boxtel R, Rosenbusch JP, Tommassen J (1997) Pore functioning of outer membrane protein PhoE of Escherichia coli: mutagenesis of the constriction loop L3. Protein Eng 10:699–706
Bonhivers M, Ghazi A, Boulanger P, Letellier L (1996) FhuA, a transporter of the Escherichia coli outer membrane, is converted into a channel upon binding of bacteriophage T5. EMBO J 15:1850–1856
Miedema H, Vrouenraets M, Wierenga J, Gillespie D, Eisenberg B, Meijberg W, Nonner W (2006) Ca2+ selectivity of a chemically modified OmpF with reduced pore volume. Biophys J 91:4392–4400
Miedema H, Vrouenraets M, Wierenga J, Eisenberg B, Schirmer T, Basle A, Meijberg W (2006) Conductance and selectivity fluctuations in D127 mutants of the bacterial porin OmpF. Eur Biophys J 36:13–22
Vrouenraets M, Wierenga J, Meijberg W, Miedema H (2006) Chemical modification of the bacterial porin OmpF: gain of selectivity by volume reduction. Biophys J 90:1202–1211
Fung BK, Hubbell WL (1978) Organization of rhodopsin in photoreceptor membranes. 1. Proteolysis of bovine rhodopsin in native membranes and the distribution of sulfhydryl groups in the fragments. Biochemistry 17:4396–4402
Degrip WJ, Vanoostrum J, Bovee-Geurts PH (1998) Selective detergent-extraction from mixed detergent/lipid/protein micelles, using cyclodextrin inclusion compounds: a novel generic approach for the preparation of proteoliposomes. Biochem J 330(Pt 2):667–674
Cornelius F (1991) Functional reconstitution of the sodium pump. Kinetics of exchange reactions performed by reconstituted Na/K-ATPase. Biochim Biophys Acta 1071:19–66
Niu L, Kim JM, Khorana HG (2002) Structure and function in rhodopsin: asymmetric reconstitution of rhodopsin in liposomes. Proc Natl Acad Sci U S A 99:13409–13412
Rigaud JL, Paternostre MT, Bluzat A (1988) Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 2. Incorporation of the light-driven proton pump bacteriorhodopsin. Biochemistry 27:2677–2688
Happe M, Teathera RM, Overath P, Knobling A, Oesterhelt D (1977) Direction of proton translocation in proteoliposomes formed from purple membrane and acidic lipids depends on the pH during reconstitution. Biochim Biophys Acta 465:415–420
Wang Z, Bai J, Xu Y (2008) The effect of charged lipids on bacteriorhodopsin membrane reconstitution and its photochemical activities. Biochem Biophys Res Commun 371:814–817
Choi HJ, Germain J, Montemagno CD (2006) Effects of different reconstitution procedures on membrane protein activities in proteopolymerosomes. Nanotechnology 17:1825–1830
Karlsson OP, Lofas S (2002) Flow-mediated on-surface reconstitution of G-protein coupled receptors for applications in surface plasmon resonance biosensors. Anal Biochem 300:132–138
Stenlund P, Babcock GJ, Sodroski J, Myszka DG (2003) Capture and reconstitution of G protein-coupled receptors on a biosensor surface. Anal Biochem 316:243–250
Bowie JU (2001) Stabilizing membrane proteins. Curr Opin Struct Biol 11:397–402
Finkelstein A (ed) (1987) Water movement through lipid bilayers, pores, and plasma membranes. Theory and reality. Wiley-Interscience, New York
Finkelstein A, Cass A (1967) Effect of cholesterol on the water permeability of thin lipid membranes. Nature 216:717–718
Overton E (1895) Über die osmotischen Eigenschaften der lebenden Pflanzen und Tierzelle. Visch Naturf Ges Zurich 40:159–201
Nagle JF, Tristram-Nagle S (2000) Structure of lipid bilayers. Biochim Biophys Acta 1469:159–195
Nagle JF, Mathai JC, Zeidel ML, Tristram-Nagle S (2008) Theory of passive permeability through lipid bilayers. J Gen Physiol 131:77–85
Mathai JC, Tristram-Nagle S, Nagle JF, Zeidel ML (2008) Structural determinants of water permeability through the lipid membrane. J Gen Physiol 131:69–76
Brockman H (1994) Dipole potential of lipid membranes. Chem Phys Lipids 73:57–79
McLaughlin S (1977) Electrostatic potentials at membrane-solution interfaces. Curr Top Membr Transp 9:71–144
Hladky SB (1979) The carrier mechanism. In: Bronner F, Kleinzeller A (eds) Current topics in membranes and transport. Academic Press, New York, pp 54–164
Barchfeld GL, Deamer DW (1985) The effect of general anaesthetics on the proton and potassium permeabilities of liposomes. Biochim Biophys Acta 819:161–169
Papahadjopoulos D (1971) Na + -K + discrimination by "pure" phospholipid membranes. Biochim Biophys Acta 241:254–259
Papahadjopoulos D, Nir S, Oki S (1972) Permeability properties of phospholipid membranes: effect of cholesterol and temperature. Biochim Biophys Acta 266:561–583
Deamer DW, Volkov AG (1995) Protein permeation of lipid bilayers. In: Disalvo EA, Simon SA (eds) Permeability and stability of lipid bilayers. CRC Press, Boca Raton, pp 161–177
Nagle JF, Morowitz HJ (1978) Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A 75:298–302
Haines TH (2001) Do sterols reduce proton and sodium leaks through lipid bilayers? Prog Lipid Res 40:299–324
Paula S, Volkov AG, Van Hoek AN, Haines TH, Deamer DW (1996) Permeation of protons, potassium ions, and small polar molecules through phospholipid bilayers as a function of membrane thickness. Biophys J 70:339–348
Haines TH (1994) Water transport across biological membranes. FEBS Lett 346:115–122
Haines TH, Liebovitch LS (1995) A molecular mechanism for the transport of water across phospholipid bilayer. In: Disalvo EA, Simon SA (eds) Permeability and stability of lipid bilayers. CRC Press, Boca Raton, pp 123–136
Rog T, Pasenkiewicz-Gierula M, Vattulainen I, Karttunen M (2009) Ordering effects of cholesterol and its analogues. Biochim Biophys Acta 1788:97–121
Kleemann G, Kellner R, Poralla K (1994) Purification and properties of the squalene-hopene cyclase from Rhodopseudomonas palustris, a purple non-sulfur bacterium producing hopanoids and tetrahymanol. Biochim Biophys Acta 1210:317–320
Sahm H, Rohmer M, Bringer-Meyer S, Sprenger GA, Welle R (1993) Biochemistry and physiology of hopanoids in bacteria. Adv Microb Physiol 35:247–273
Gliozzi A, Relini A, Lee-Gau Chong P (2002) Structure and permeability properties of biomimetic membranes of archaeal bolaform tetraether lipids. J. Membr. Sci. 206:131–147
Sikkema J, de Bont JA, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59:201–222
Valtersson C, van Duyn G, Verkleij AJ, Chojnacki T, de Kruijff B, Dallner G (1985) The influence of dolichol, dolichol esters, and dolichyl phosphate on phospholipid polymorphism and fluidity in model membranes. J Biol Chem 260:2742–2751
Monti JA, Christian ST, Schutzbach JS (1987) Effects of dolichol on membrane permeability. Biochim Biophys Acta 905:133–142
Katsikas H, Quinn PJ (1983) Fluorescence probe studies of the distribution of ubiquinone homologues in bilayers of dipalmitoylglycerophosphocholine. Eur J Biochem 131:607–612
Fitch CD, Folkers K (1967) Coenzyme Q and the stability of biological membranes. Biochem Biophys Res Commun 26:128–131
Stillwell W, Dallman T, Dumaual AC, Crump FT, Jenski LJ (1996) Cholesterol versus alpha-tocopherol: effects on properties of bilayers made from heteroacid phosphatidylcholines. Biochemistry 35:13353–13362
Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM (2008) Science and technology for water purification in the coming decades. Nature 452:301–310
Skou JC, Esmann M (1992) The Na,K-ATPase. J Bioenerg Biomembr 24:249–261
Seifert K, Fendler K, Bamberg E (1993) Charge transport by ion translocating membrane proteins on solid supported membranes. Biophys J 64:384–391
Pintschovius J, Fendler K, Bamberg E (1999) Charge translocation by the Na+/K+-ATPase investigated on solid supported membranes: cytoplasmic cation binding and release. Biophys J 76:827–836
Pintschovius J, Fendler K (1999) Charge translocation by the Na+/K+-ATPase investigated on solid supported membranes: rapid solution exchange with a new technique. Biophys J 76:814–826
Zebrowska A, Krysinski P (2004) Incorporation of Na(+), K(+)-ATP-ase into the thiolipid biomimetic assemblies via the fusion of proteoliposomes. Langmuir 20:11127–11133
Tadini-Buoninsegni F, Bartolommei G, Moncelli MR, Fendler K (2008) Charge transfer in P-type ATPases investigated on planar membranes. Arch Biochem Biophys 476:75–86
Tadini Buoninsegni F, Bartolommei G, Moncelli MR, Inesi G, Guidelli R (2004) Time-resolved charge translocation by sarcoplasmic reticulum Ca-ATPase measured on a solid supported membrane. Biophys J 86:3671–3686
Nielsen CH, Abdali S, Lundbæk JA, Cornelius F (2005) Raman spectroscopy of conformational changes in membrane-bound sodium potassium ATPase. Spectroscopy 22:52–63
Mulkidjanian AY, Makarova KS, Galperin MY, Koonin EV (2007) Inventing the dynamo machine: the evolution of the F-type and V-type ATPases. Nat Rev Microbiol 5:892–899
Boyer PD (1997) The ATP synthase-a splendid molecular machine. Annu Rev Biochem 66:717–749
Jefferies KC, Cipriano DJ, Forgac M (2008) Function, structure and regulation of the vacuolar (H+)-ATPases. Arch Biochem Biophys 476:33–42
Cipriano DJ, Wang Y, Bond S, Hinton A, Jefferies KC, Qi J, Forgac M (2008) Structure and regulation of the vacuolar ATPases. Biochim Biophys Acta 1777:599–604
Ueno H, Suzuki T, Kinosita K Jr, Yoshida M (2005) ATP-driven stepwise rotation of FoF1-ATP synthase. Proc Natl Acad Sci U S A 102:1333–1338
Tutus M, Rossetti FF, Schneck E, Fragneto G, Förster F, Ricther R, Nawroth T, Tanaka M (2008) Orientation-selective incorporation of transmemrbane F0F1 ATP synthase complex from Micrococcus luteus in polymer-supported membranes. Macromol Biosci 8:1034–1043
Naumann R, Baumgart T, Graber P, Jonczyk A, Offenhausser A, Knoll W (2002) Proton transport through a peptide-tethered bilayer lipid membrane by the H(+)-ATP synthase from chloroplasts measured by impedance spectroscopy. Biosens Bioelectron 17:25–34
Knoll W, Frank CW, Heibel C, Naumann R, Offenhausser A, Ruhe J, Schmidt EK, Shen WW, Sinner A (2000) Functional tethered lipid bilayers. J Biotechnol 74:137–158
Mulkidjanian AY, Dibrov P, Galperin MY (2008) The past and present of sodium energetics: may the sodium-motive force be with you. Biochim Biophys Acta 1777:985–992
Mulkidjanian AY, Galperin MY, Koonin EV (2009) Co-evolution of primordial membranes and membrane proteins. Trends Biochem Sci 34:206–215
LaVan DA, Cha JN (2006) Approaches for biological and biomimetic energy conversion. Proc Natl Acad Sci U S A 103:5251–5255
Oesterhelt D, Stoeckenius W (1973) Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A 70:2853–2857
Schobert B, Lanyi JK (1982) Halorhodopsin is a light-driven chloride pump. J Biol Chem 257:10306–10313
Essen LO (2002) Halorhodopsin: light-driven ion pumping made simple? Curr Opin Struct Biol 12:516–522
Bamberg E, Tittor J, Oesterhelt D (1993) Light-driven proton or chloride pumping by halorhodopsin. Proc Natl Acad Sci U S A 90:639–643
Kayushin LP, Skulachev VP (1974) Bacteriorhodopsin as an electrogenic proton pump: reconstitution of bacteriorhodopsin proteoliposomes generating delta psi and delta pH. FEBS Lett 39:39–42
Oesterhelt D, Schuhmann L (1974) Reconstitution of bacteriorhodopsin. FEBS Lett 44:262–265
Ganea C, Tittor J, Bamberg E, Oesterhelt D (1998) Chloride- and pH-dependent proton transport by BR mutant D85N. Biochim Biophys Acta 1368:84–96
Horn C, Steinem C (2005) Photocurrents generated by bacteriorhodopsin adsorbed on nano-black lipid membranes. Biophys J 89:1046–1054
Varo G (2000) Analogies between halorhodopsin and bacteriorhodopsin. Biochim Biophys Acta 1460:220–229
Duschl A, McCloskey MA, Lanyi JK (1988) Functional reconstitution of halorhodopsin. Properties of halorhodopsin-containing proteoliposomes. J Biol Chem 263:17016–17022
Bamberg E, Hegemann P, Oesterhelt D (1984) Reconstitution of halorhodopsin in black lipid membranes. Prog Clin Biol Res 164:73–79
Gruia AD, Bondar AN, Smith JC, Fischer S (2005) Mechanism of a molecular valve in the halorhodopsin chloride pump. Structure 13:617–627
Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TL, Petersen J, Andersen JP, Vilsen B, Nissen P (2007) Crystal structure of the sodium-potassium pump. Nature 450:1043–1049
Shinoda T, Ogawa H, Cornelius F, Toyoshima C (2009) Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature 459:446–450
Toyoshima C, Nakasako M, Nomura H, Ogawa H (2000) Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature 405:647–655
Toyoshima C, Inesi G (2004) Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu Rev Biochem 73:269–292
Accardi A, Miller C (2004) Secondary active transport mediated by a prokaryotic homologue of ClC Cl- channels. Nature 427:803–807
Scheiner-Bobis G (1998) Ion-transporting ATPases as ion channels. Naunyn Schmiedebergs Arch Pharmacol 357:477–482
Gadsby DC (2009) Ion channels versus ion pumps: the principal difference, in principle. Nat Rev Mol Cell Biol
Ashcroft F, Gadsby D, Miller C (2009) Introduction. The blurred boundary between channels and transporters. Philos Trans R Soc Lond B Biol Sci 364:145–147
Kedem O, Katchalsky A (1958) Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta 27:229–246
Kedem O, Katchalsky A (1961) A physical interpretation of the phenomenological coefficients of membrane permeability. J Gen Physiol 45:143–179
Goldman DE (1943) Potential, impedance and rectification in membranes. J. Gen. Physiol. 27:37–60
Chen D, Eisenberg R (1993) Charges, currents, and potentials in ionic channels of one conformation. Biophys J 64:1405–1421
Chen DP, Eisenberg RS (1993) Flux, coupling, and selectivity in ionic channels of one conformation. Biophys J 65:727–746
Miller C (1999) Ionic hopping defended. J Gen Physiol 113:783–787
Chung SH, Kuyucak S (2002) Recent advances in ion channel research. Biochim Biophys Acta 1565:267–286
Roux B (2005) Ion conduction and selectivity in K(+) channels. Annu Rev Biophys Biomol Struct 34:153–171
Edwards S, Corry B, Kuyucak S, Chung SH (2002) Continuum electrostatics fails to describe ion permeation in the gramicidin channel. Biophys J 83:1348–1360
Allen TW, Andersen OS, Roux B (2006) Molecular dynamics - potential of mean force calculations as a tool for understanding ion permeation and selectivity in narrow channels. Biophys Chem 124:251–267
Allen TW, Bastug T, Kuyucak S, Chung SH (2003) Gramicidin A channel as a test ground for molecular dynamics force fields. Biophys J 84:2159–2168
Andersen OS, Koppe RE II, Roux B (2007) Gramicidin channels: versatile tools. In: Chung SH, Andersen OS, Krishnamurthy V (eds) Biological membrane ion channels. Springer, New York, pp 33–80
Cukierman S (2000) Proton mobilities in water and in different stereoisomers of covalently linked gramicidin A channels. Biophys J 78:1825–1834
Koeppe RE II, Andersen OS (1996) Engineering the gramicidin channel. Annu Rev Biophys Biomol Struct 25:231–258
Chung SH, Andersen OS, Krishnamurthy V (eds) (2007) Biological membrane ion channels. Dynamics, Structure and Applications, Springer, New York
Huang L-YM, Catterall WA, Ehrenstein G (1978) Selectivity of cations and nonelectrolytes for acetylcholine-activated channels in cultured muscle cells. J Gen Physiol 71:397–410
Unwin N (2005) Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol 346:967–989
Boheim G, Hanke W, Barrantes FJ, Eibl H, Sakmann B, Fels G, Maelicke A (1981) Agonist-activated ionic channels in acetylcholine receptor reconstituted into planar lipid bilayers. Proc Natl Acad Sci U S A 78:3586–3590
Schmidt EK, Liebermann T, Kreiter M, Jonczyk A, Naumann R, Offenhausser A, Neumann E, Kukol A, Maelicke A, Knoll W (1998) Incorporation of the acetylcholine receptor dimer from Torpedo californica in a peptide supported lipid membrane investigated by surface plasmon and fluorescence spectroscopy. Biosens Bioelectron 13:585–591
Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280:69–77
Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R (2002) Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417:515–522
Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R (2003) X-ray structure of a voltage-dependent K+ channel. Nature 423:33–41
Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA (2003) Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300:1922–1926
MacKinnon R (2003) Potassium channels. FEBS Lett 555:62–65
Sexton LT, Horne LP, Martin CR (2007) Developing synthetic conical nanopores for biosensing applications. Mol Biosyst 3:667–685
Jeon YJ, Kim H, Jon S, Selvapalam N, Oh DH, Seo I, Park CS, Jung SR, Koh DS, Kim K (2004) Artificial ion channel formed by cucurbit[n]uril derivatives with a carbonyl group fringed portal reminiscent of the selectivity filter of K+ channels. J Am Chem Soc 126:15944–15945
Terrettaz S, Follonier S, Makohliso S, Vogel H (2009) A synthetic membrane protein in tethered lipid bilayers for immunosensing in whole blood. J Struct Biol
Gandhi CS, Clark E, Loots E, Pralle A, Isacoff EY (2003) The orientation and molecular movement of a k(+) channel voltage-sensing domain. Neuron 40:515–525
Laine M, Lin MC, Bannister JP, Silverman WR, Mock AF, Roux B, Papazian DM (2003) Atomic proximity between S4 segment and pore domain in Shaker potassium channels. Neuron 39:467–481
Blaustein RO, Miller C (2004) Ion channels: shake, rattle or roll? Nature 427:499–500
Long SB, Campbell EB, Mackinnon R (2005) Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309:897–903
Long SB, Campbell EB, Mackinnon R (2005) Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science 309:903–908
Dhoke MA, Ladha PJ, Boerio FJ, Lessard LB, Malinowska DH, Cuppoletti J, Wieczorek DS (2005) Porous membranes for reconstitution of ion channels. Biochim Biophys Acta 1716:117–125
Matsuno N, Murawsky M, Ridgeway J, Cuppoletti J (2004) Solid support membranes for ion channel arrays and sensors: application to rapid screening of pharmacological compounds. Biochim Biophys Acta 1665:184–190
Blunck R, McGuire H, Hyde HC, Bezanilla F (2008) Fluorescence detection of the movement of single KcsA subunits reveals cooperativity. Proc Natl Acad Sci U S A 105:20263–20268
DeCoursey TE (2008) Voltage-gated proton channels: what’s next? J Physiol 586:5305–5324
DeCoursey TE (2008) Voltage-gated proton channels. Cell Mol Life Sci 65:2554–2573
Cherny VV, Murphy R, Sokolov V, Levis RA, DeCoursey TE (2003) Properties of single voltage-gated proton channels in human eosinophils estimated by noise analysis and by direct measurement. J Gen Physiol 121:615–628
Demaurex N, Grinstein S, Jaconi M, Schlegel W, Lew DP, Krause KH (1993) Proton currents in human granulocytes: regulation by membrane potential and intracellular pH. J Physiol 466:329–344
Schilling T, Gratopp A, DeCoursey TE, Eder C (2002) Voltage-activated proton currents in human lymphocytes. J Physiol 545:93–105
Tombola F, Ulbrich MH, Isacoff EY (2008) The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron 58:546–556
Koch HP, Kurokawa T, Okochi Y, Sasaki M, Okamura Y, Larsson HP (2008) Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci U S A 105:9111–9116
Lee SY, Letts JA, Mackinnon R (2008) Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc Natl Acad Sci U S A 105:7692–7695
Sasaki M, Takagi M, Okamura Y (2006) A voltage sensor-domain protein is a voltage-gated proton channel. Science 312:589–592
Ramsey IS, Moran MM, Chong JA, Clapham DE (2006) A voltage-gated proton-selective channel lacking the pore domain. Nature 440:1213–1216
Starace DM, Bezanilla F (2004) A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature 427:548–553
Starace DM, Bezanilla F (2001) Histidine scanning mutagenesis of basic residues of the S4 segment of the shaker k+ channel. J Gen Physiol 117:469–490
Lee SY, Letts JA, MacKinnon R (2009) Functional reconstitution of purified human Hv1 H+ channels. J Mol Biol 387:1055–1060
Agre P, Sasaki S, Chrispeels MJ (1993) Aquaporins: a family of water channel proteins. Am J Physiol 265:F461
Gonen T, Walz T (2006) The structure of aquaporins. Q Rev Biophys 39:361–396
Fu D, Lu M (2007) The structural basis of water permeation and proton exclusion in aquaporins. Mol Membr Biol 24:366–374
Ishibashi K (2006) Aquaporin subfamily with unusual NPA boxes. Biochim Biophys Acta 1758:989–993
Verkman AS (2005) More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci 118:3225–3232
Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA (2002) Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82:205–244
Borgnia MJ, Agre P (2001) Reconstitution and functional comparison of purified GlpF and AqpZ, the glycerol and water channels from Escherichia coli. Proc Natl Acad Sci U S A 98:2888–2893
Calamita G, Bishai WR, Preston GM, Guggino WB, Agre P (1995) Molecular cloning and characterization of AqpZ, a water channel from Escherichia coli. J Biol Chem 270:29063–29066
Fu D, Libson A, Miercke LJ, Weitzman C, Nollert P, Krucinski J, Stroud RM (2000) Structure of a glycerol-conducting channel and the basis for its selectivity. Science 290:481–486
Nernst W (1909) Zur Theorie des electrischen Reizes. Pfluegers Arch. Physiol 122:275–314
Anthony TL, Brooks HL, Boassa D, Leonov S, Yanochko GM, Regan JW, Yool AJ (2000) Cloned human aquaporin-1 is a cyclic GMP-gated ion channel. Mol Pharmacol 57:576–588
Cooper GJ, Boron WF (1998) Effect of PCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am J Physiol 275:C1481–C1486
Herrera M, Hong NJ, Garvin JL (2006) Aquaporin-1 transports NO across cell membranes. Hypertension 48:157–164
Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL (2001) Transport of NH(3)/NH in oocytes expressing aquaporin-1. Am J Physiol Renal Physiol 281:F255–F263
Yasui M, Kwon TH, Knepper MA, Nielsen S, Agre P (1999) Aquaporin-6: an intracellular vesicle water channel protein in renal epithelia. Proc Natl Acad Sci U S A 96:5808–5813
Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, Rosen BP (2002) Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci U S A 99:6053–6058
Stroud RM, Miercke LJ, O’Connell J, Khademi S, Lee JK, Remis J, Harries W, Robles Y, Akhavan D (2003) Glycerol facilitator GlpF and the associated aquaporin family of channels. Curr Opin Struct Biol 13:424–431
Stroud RM, Savage D, Miercke LJ, Lee JK, Khademi S, Harries W (2003) Selectivity and conductance among the glycerol and water conducting aquaporin family of channels. FEBS Lett 555:79–84
Verkman AS (2002) Does aquaporin-1 pass gas? An opposing view. J Physiol 542:31
Wu B, Beitz E (2007) Aquaporins with selectivity for unconventional permeants. Cell Mol Life Sci 64:2413–2421
Bienert GP, Schussler MD, Jahn TP (2008) Metalloids: essential, beneficial or toxic? Major intrinsic proteins sort it out. Trends Biochem Sci 33:20–26
Khandelia H, Jensen MO, Mouritsen OG (2009) To gate or not to gate: using molecular dynamics simulations to morph gated plant aquaporins into constitutively open conformations. J Phys Chem B 113:5239–5244
Nemeth-Cahalan KL, Hall JE (2000) pH and calcium regulate the water permeability of aquaporin 0. J Biol Chem 275:6777–6782
Nemeth-Cahalan KL, Kalman K, Hall JE (2004) Molecular basis of pH and Ca2+ regulation of aquaporin water permeability. J Gen Physiol 123:573–580
Johansson I, Larsson C, Ek B, Kjellbom P (1996) The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell 8:1181–1191
Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P (1998) Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10:451–459
Zeuthen T, Klaerke DA (1999) Transport of water and glycerol in aquaporin 3 is gated by H(+). J Biol Chem 274:21631–21636
Yasui M, Hazama A, Kwon TH, Nielsen S, Guggino WB, Agre P (1999) Rapid gating and anion permeability of an intracellular aquaporin. Nature 402:184–187
Nernst W (1904) Theorie der Reaktionsgeschwindigkeit in heterogenen Systemen. Z Phys Chem (Leipzig) 47:52–55
Schulman JH, Teorell T (1938) On the boundary layer at membrane and monolayer interfaces. Trans Faraday Soc 34:1337
Pearce GK (2008) UF/MF pre-treatment to RO in seawater and wastewater reuse applications: a comparison of energy costs
Byrne W (2002) Reverse osmosis. Tall Oaks Publishing Inc., Littleton, CO
Meltzer TH (1993) High-purity water. Tall Oaks Publishing, Littleton, CO
Macdonald AG (2002) Ion channels under high pressure. Comp Biochem Physiol A Mol Integr Physiol 131:587–593
Griepernau B, Bockmann RA (2008) The influence of 1-alkanols and external pressure on the lateral pressure profiles of lipid bilayers. Biophys J 95:5766–5778
Loeb S, Norman RS (1975) Osmotic power plants. Science 189:654–655
Skilhagen SE, Dugstad JE, Aaberg RJ (2008) Osmotic power - power production based on the osmotic pressure difference between waters with varying salt gradients. Desalination 220:476–482
Cath TY, Childress AE, Elimelech M (2006) Forward osmosis: principles, applications and recent developments. J Membr Sci 281:70–87
Kessler JO, Moody CD (1976) Drinking water from sea water by forward osmosis. Desalination 18:297–306
McCutcheon JR, McGinnis RL, Elimelech M (2006) Desalination by a novel ammonia-carbon dioxide forward osmosis process: influence of draw and feed solution concentrations on process performance. J Membr Sci 278:114–123
Sleytr U, Bayley H, Sára M, Breitwieser A, Küpcü S, Mader C, Weigert S, Unger F, Messner P, Jahn-Schmid B, Schuster B, Pum D, Douglas K, Clark N, Moore J, Winningham T, Levy S, Frithsen I, Pankovc J, Beale P, Gillis H, Choutov D, Martin KP (1997) Applications of S-layers. FEMS Microbiol Rev 29:151–175
Szewczykowski P (2009) Nano-porous materials from diblock copolymers and their membrane application. PhD Thesis, Department of Chemical and Biochemical Engineering, Technical University of Denmark, Lyngby
Walter A, Gutknecht J (1986) Permeability of small nonelectrolytes through lipid bilayer membranes. J Membrane Biol 77:255–264
Flewelling RF, Hubbell WL (1986) Hydrophobic ion interactions with membranes. Thermodynamic analysis of tetraphenylphosphonium binding to vesicles. Biophys J 49:531–540
Hauser H, Oldani D, Phillips MC (1973) Mechanism of ion escape from phosphatidylcholine and phosphatidylserine single bilayer vesicles. Biochemistry 12:4507–4517
Gutknecht J (1984) Proton/hydroxide conductance through lipid bilayer membranes. J Membr Biol 82:105–112
Acknowledgements
This work was supported through MEMBAQ, a specific targeted research project (STREP), by the European Commission under the Sixth Framework Programme (NMP4-CT-2006-033234), though Watermembrane, by The Danish National Advanced Technology Foundation (023-2007-1), and by a grant from The Danish National Research Foundation to QuP. I thank my colleagues in MEMBAQ, Watermembrane, QuP, and Aquaporin A/S for helpful discussions and the reviewers for their constructive comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nielsen, C.H. Biomimetic membranes for sensor and separation applications. Anal Bioanal Chem 395, 697–718 (2009). https://doi.org/10.1007/s00216-009-2960-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-2960-0