Abstract
Rationale
Obsessive-compulsive disorder (OCD) is a psychiatric disorder characterized by intrusive obsessive thoughts and/or compulsive behaviors. Currently, serotonin reuptake inhibitors (SRIs) provide the only pharmacological monotherapy for OCD, but response rates are insufficient. Ketamine, a noncompetitive NMDA receptor antagonist, was reported to have rapid, sustained therapeutic effects in OCD patients. However, the mechanisms remain unknown.
Objectives
Here, we aimed to provide a platform for investigating mechanisms underlying anti-OCD effects of ketamine treatment by assessing whether ketamine pretreatment could alleviate 5-HT1B receptor (5-HT1BR)-induced OCD-like behavior in mice.
Methods
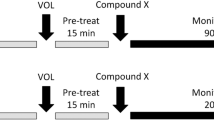
We assessed whether acute ketamine (0, 3, 10, 30 mg/kg), administered at two pretreatment time points (30 min, 24 h), would modulate 5-HT1BR-induced OCD-like behavior in mice. Behavioral measures were perseverative hyperlocomotion in the open field and deficits in prepulse inhibition (PPI) induced by acute pharmacological 5-HT1BR challenge.
Results
Three milligrams per kilogram of ketamine reduced 5-HT1BR-induced perseverative hyperlocomotion, but not PPI deficits, 24 h postinjection. In contrast, higher doses of ketamine were either ineffective (10 mg/kg) or exacerbated (30 mg/kg) 5-HT1BR-induced perseverative hyperlocomotion 30 min postinjection. At 24 h postinjection, 30 mg/kg ketamine reduced perseverative hyperlocomotion across all groups.
Conclusions
Our results suggest that the 5-HT1BR-induced model of OCD-like behavior is sensitive to a low dose of ketamine, a potential fast-acting anti-OCD treatment, and may provide a tool for studying mechanisms underlying the rapid therapeutic effects of ketamine in OCD patients.


Similar content being viewed by others
References
Ahmari SE, Risbrough VB, Geyer MA, Simpson HB (2012) Impaired sensorimotor gating in unmedicated adults with obsessive-compulsive disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 37:1216–1223.
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub
Autry AE, Adachi M, Nosyreva E et al (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95.
Babyak MA (2004) What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med 66:411– 421.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354
Bloch MH, Wasylink S, Landeros-Weisenberger A et al (2012) Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry 72:964–970.
Chan M-H, Chiu P-H, Lin C-Y, Chen H-H (2012) Inhibition of glycogen synthase kinase-3 attenuates psychotomimetic effects of ketamine. Schizophr Res 136:96–103.
Chan M-H, Chiu P-H, Sou J-H, Chen H-H (2008) Attenuation of ketamine-evoked behavioral responses by mGluR5 positive modulators in mice. Psychopharmacology 198:141–148.
Chaturvedi HK, Dinesh C, Bapna JS (1999) Effect of NMDA receptor antagonists in forced swimming test and its modification by antidepressants. Indian J Pharm 31:104
Choi I-S, Cho J-H, An C-H et al (2012) 5-HT(1B) receptors inhibit glutamate release from primary afferent terminals in rat medullary dorsal horn neurons. Br J Pharmacol 167:356–367.
Chowdhury GMI, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G (2012) 1H-[13C]-Nuclear magnetic resonance spectroscopy measures of ketamine’s effect on amino acid neurotransmitter metabolism. Biol Psychiatry 71:1022–1025.
de Brouwer G, Fick A, Harvey BH, Wolmarans DW (2018) A critical inquiry into marble-burying as a preclinical screening paradigm of relevance for anxiety and obsessive-compulsive disorder: mapping the way forward. Cogn Affect Behav Neurosci 19:1–39.
de Oliveira L, Spiazzi CM, Bortolin T et al (2009) Different sub-anesthetic doses of ketamine increase oxidative stress in the brain of rats. Prog Neuro- Psychopharmacol Biol Psychiatry 33:1003–1008.
Dold M, Aigner M, Lanzenberger R, Kasper S (2015) Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: an update meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP.
Douma TN, Millan MJ, Boulay D, Griebel G, Verdouw PM, Westphal KG, Olivier B, Groenink L (2014) CRF1 receptor antagonists do not reverse pharmacological disruption of prepulse inhibition in rodents. Psychopharmacology 231:1289–1303.
du Jardin KG, Liebenberg N, Cajina M, Müller HK, Elfving B, Sanchez C, Wegener G (2017) S-Ketamine mediates its acute and sustained antidepressant-like activity through a 5-HT1B receptor dependent mechanism in a genetic rat model of depression. Front Pharmacol 8:978.
Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, Kirkwood K, aan het Rot M, Lapidus KA, Wan LB, Iosifescu D, Charney DS (2014) Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry 71:681–688.
Fineberg NA, Brown A, Reghunandanan S, Pampaloni I (2012) Evidence-based pharmacotherapy of obsessive-compulsive disorder. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP 15:1173–1191.
Fisher RA (1934) IV: Tests of goodness of fit, independence and homogeneity; WITH TABLE OF X2. In: Statistical Methods for Research Workers, 5th edn. Oliver and Boyd, pp 103–105
Fraga DB, Olescowicz G, Moretti M, Siteneski A, Tavares MK, Azevedo D, Colla ARS, Rodrigues ALS (2018) Anxiolytic effects of ascorbic acid and ketamine in mice. J Psychiatr Res 100:16–23.
Franceschelli A, Sens J, Herchick S et al (2015) Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience 290:49–60.
Gerhard DM, Wohleb ES, Duman RS (2016) Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today 21:454–464.
Ghasemi M, Raza M, Dehpour AR (2010) NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. J Psychopharmacol Oxf Engl 24:585–594.
Gideons ES, Kavalali ET, Monteggia LM (2014) Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc Natl Acad Sci U S A 111:8649–8654.
Greist JH, Jefferson JW, Kobak KA, Katzelnick DJ, Serlin RC (1995) Efficacy and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder. A meta-analysis. Arch Gen Psychiatry 52:53–60
Gross-Isseroff R, Cohen R, Sasson Y, Voet H, Zohar J (2004) Serotonergic dissection of obsessive compulsive symptoms: a challenge study with mchlorophenylpiperazine and sumatriptan. Neuropsychobiology 50:200–205.
Guo J-D, O’Flaherty BM, Rainnie DG (2017) Serotonin gating of cortical and thalamic glutamate inputs onto principal neurons of the basolateral amygdala. Neuropharmacology 126:224–232.
Hayase T, Yamamoto Y, Yamamoto K (2006) Behavioral effects of ketamine and toxic interactions with psychostimulants. BMC Neurosci 7:25.
Ho EV, Thompson SL, Katzka WR, Sharifi MF, Knowles JA, Dulawa SC (2016) Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive 10.1007/s00213-019-05397-8 Ho EV, Thompson SL, Katzka WR, Sharifi MF, Knowles JA, Dulawa SC (2016) Clinically effective OCD treatment prevents 5-HT1B receptor-induced repetitive behavior and striatal activation. Psychopharmacology 233:57–70.
Holubova K, Kleteckova L, Skurlova M, Ricny J, Stuchlik A, Vales K (2016) Rapamycin blocks the antidepressant effect of ketamine in task-dependent manner. Psychopharmacology 233:2077–2097.
Hou Y, Zhang H, Xie G, Cao X, Zhao Y, Liu Y, Mao Z, Yang J, Wu C (2013) Neuronal injury, but not microglia activation, is associated with ketamine-induced experimental schizophrenic model in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 45:107–116.
Imre G, Fokkema DS, Boer JAD, Ter Horst GJ (2006) Dose–response characteristics of ketamine effect on locomotion, cognitive function and central neuronal activity. Brain Res Bull 69:338–345.
Issaria Y, Jakubovski E, Bartley CA et al (2016) Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a metaanalysis. J Clin Psychiatry 77:e605–e611.
Kieschnick R, McCullough BD (2003) Regression analysis of variates observed on (0, 1): percentages, proportions and fractions. Stat Model Int J 3:193–213. https://doi.org/10.1191/1471082X03st053oa
Koran LM, Pallanti S, Quercioli L (2001) Sumatriptan, 5-HT(1D) receptors and obsessive-compulsive disorder. Eur Neuropsychopharmacol J Eur Coll Neuropsychopharmacol 11:169–172
Li N, Liu R-J, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS (2011) Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761.
Li X, Martinez-Lozano Sinues P, Dallmann R et al (2015) Drug pharmacokinetics determined by real-time analysis of mouse breath. Angew Chem Int Ed 54:7815–7818.
Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA (2003) Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 117:697–706
Lu CW, Lin TY, Huang SK, Wang SJ (2018) 5-HT1B receptor agonist CGS12066 presynaptically inhibits glutamate release in rat hippocampus. Prog Neuro- Psychopharmacol Biol Psychiatry 86:122–130.
Maeng S, Zarate CA, Du J et al (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole- 4-propionic acid receptors. Biol Psychiatry 63:349–352.
McGowan JC, LaGamma CT, Lim SC, Tsitsiklis M, Neria Y, Brachman RA, Denny CA (2017) Prophylactic ketamine attenuates learned fear. Neuropsychopharmacology 42:1577–1589.
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927
Montgomery SA, Manceaux A (1992) Fluvoxamine in the treatment of obsessive compulsive disorder. Int Clin Psychopharmacol:5–10
Oberlander C, Demassey Y, Verdu A, van de Velde D, Bardelay C (1987) Tolerance to the serotonin 5-HT1 agonist RU 24969 and effects on dopaminergic behaviour. Eur J Pharmacol 139:205–214
Ossato A, Bilel S, Gregori A, Talarico A, Trapella C, Gaudio RM, de-Giorgio F, Tagliaro F, Neri M, Fattore L, Marti M (2018) Neurological, sensorimotor and cardiorespiratory alterations induced by methoxetamine, ketamine and phencyclidine in mice. Neuropharmacology 141:167–180.
Paulus MP, Geyer MA (1991a) A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology 104:6–16
Paulus MP, Geyer MA (1991b) A scaling approach to find order parameters quantifying the effects of dopaminergic agents on unconditioned motor activity in rats. Prog Neuro-Psychopharmacol Biol Psychiatry 15:903–919.
Popik P, Hołuj M, Kos T, Nowak G, Librowski T, Sałat K (2017) Comparison of the psychopharmacological effects of tiletamine and ketamine in rodents. Neurotox Res 32:544–554.
Popik P, Kos T, Sowa-Kućma M, Nowak G (2008) Lack of persistent effects of ketamine in rodent models of depression. Psychopharmacology 198:421–430.
Radford KD, Park TY, Lee BH et al (2017) Dose-response characteristics of intravenous ketamine on dissociative stereotypy, locomotion, sensorimotor gating, and nociception in male Sprague-Dawley rats. Pharmacol Biochem Behav 153:130–140.
Razoux F, Garcia R, Léna I (2007) Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 32:719–727.
Refsgaard LK, Pickering DS, Andreasen JT (2017) Investigation of antidepressant-like and anxiolytic-like actions and cognitive and motor side effects of four Nmethyl-D-aspartate receptor antagonists in mice. Behav Pharmacol 28:37–47.
Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, Flood P, Simpson HB (2013) Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 38:2475–2483. 10.1007/s00213-019-05397-8 obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 38:2475–2483.
Rodriguez CI, Wheaton M, Zwerling J et al (2016) Can exposure-based CBT extend the effects of intravenous ketamine in obsessive-compulsive disorder? an open-label trial. J Clin Psychiatry 77:408–409.
Ruscio AM, Stein DJ, Chiu WT, Kessler RC (2010) The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 15:53–63.
Sałat K, Siwek A, Starowicz G et al (2015) Antidepressant-like effects of ketamine, norketamine and dehydronorketamine in forced swim test: Role of activity at NMDA receptor. Neuropharmacology 99:301–307.
Shanahan NA, Holick Pierz KA, Masten VL, Waeber C, Ansorge M, Gingrich JA, Geyer MA, Hen R, Dulawa SC (2009) Chronic reductions in serotonin transporter function prevent 5-HT1B-induced behavioral effects in mice. Biol Psychiatry 65:401–408.
Shanahan NA, Velez LP, Masten VL, Dulawa SC (2011) Essential role for orbitofrontal serotonin 1B receptors in obsessive-compulsive disorder-like behavior and serotonin reuptake inhibitor response in mice. Biol Psychiatry 70:1039–1048.
Smithson M, Verkuilen J (2006) A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol Methods 11:54–71.
Soumier A, Carter RM, Schoenfeld TJ, Cameron HA (2016) New hippocampal neurons mature rapidly in response to ketamine but are not required for its acute antidepressant effects on neophagia in rats. eNeuro 3:.
Stein DJ, Van Heerden B, Wessels CJ et al (1999) Single photon emission computed tomography of the brain with Tc-99 m HMPAO during sumatriptan challenge in obsessive-compulsive disorder: investigating the functional role of the serotonin auto-receptor. Prog Neuro-Psychopharmacol Biol Psychiatry 23:1079–1099
Stern ER, Taylor SF (2014) Cognitive neuroscience of obsessive-compulsive disorder. Psychiatr Clin North Am 37:337–352.
Thelen C, Flaherty E, Saurine J, Sens J, Mohamed S, Pitychoutis PM (2019) Sex differences in the temporal neuromolecular and synaptogenic effects of the rapidacting antidepressant drug ketamine in the mouse brain. Neuroscience 398:182–192.
Thompson SL, Dulawa SC (2019) Dissecting the roles of β-arrestin2 and GSK-3 signaling in 5-HT1BR-mediated perseverative behavior and prepulse inhibition deficits in mice. PLoS One 14:e0211239.
Thompson SL, Dulawa SC (2017) Pharmacological and behavioral rodent models of OCD. In: Pittenger C (ed) Obsessive-Compulsive Disorder: Phenomenology, Pathophysiology, and Treatment. Oxford University Press, pp 385–400
Tosta CL, Silote GP, Fracalossi MP, Sartim AG, Andreatini R, Joca SRL, Beijamini V (2019) S-ketamine reduces marble burying behaviour: involvement of ventromedial orbitofrontal cortex and AMPA receptors. Neuropharmacology 144:233–243.
Veale D, Miles S, Smallcombe N, Ghezai H, Goldacre B, Hodsoll J (2014) Atypical antipsychotic augmentation in SSRI treatment refractory obsessivecompulsive disorder: a systematic review and meta-analysis. BMC Psychiatry 14:317.
Wu SY, Wang MY, Dun NJ (1991) Serotonin via presynaptic 5-HT1 receptors attenuates synaptic transmission to immature rat motoneurons in vitro. Brain Res 554:111–121.
Yamanaka H, Yokoyama C, Mizuma H, Kurai S, Finnema SJ, Halldin C, Doi H, Onoe H (2014) A possible mechanism of the nucleus accumbens and ventral pallidum 5-HT1B receptors underlying the antidepressant action of ketamine: a PET study with macaques. Transl Psychiatry 4:e342.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486.
Zanos P, Nelson ME, Highland JN, et al (2017) A negative allosteric modulator for α5 subunit-containing GABA receptors exerts a rapid and persistent antidepressant-like action without the side effects of the NMDA receptor antagonist ketamine in mice. eNeuro 4:.
Zanos P, Piantadosi SC, Wu H-Q et al (2015) The prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/GlycineB-Site Inhibition. J Pharmacol Exp Ther 355:76–85.
Zarate CA, Singh JB, Carlson PJ et al (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864.
Funding and disclosures
This work was supported by an IMHRO Rising Star Depression Research Award in Memory of George Largay, a NARSAD Independent Investigator Award , and R21MH115395 to SD, and training grants: T32 GM07839 and T32 DA07255 to SLT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 137 kb)
Rights and permissions
About this article
Cite this article
Thompson, S.L., Welch, A.C., Iourinets, J. et al. Ketamine induces immediate and delayed alterations of OCD-like behavior. Psychopharmacology 237, 627–638 (2020). https://doi.org/10.1007/s00213-019-05397-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05397-8