Abstract
Rationale
Unlike other drugs of abuse, Δ9-tetrahydrocanabinol (THC) is generally aversive in rodent conditioned place preference models, but little is known about how stress may modify THC affective properties.
Objective
We evaluate the potential of footshock stress to enhance the rewarding effects of THC and the fatty acid amide hydrolase inhibitor, URB597, as it has been shown to enhance their anxiolytic effects.
Materials and methods
The effect of footshock stress 24 h prior to each conditioning trial on the rewarding/aversive effects of THC (1, 0.1, 0.5 mg/kg, ip) and URB597 (0.3 mg/kg, ip) was evaluated in an unbiased place conditioning procedure in rats. Subsequently, the same stressor was given immediately prior to conditioning with THC (1 and 0.1 mg/kg). Locomotor activity was also measured during conditioning.
Results
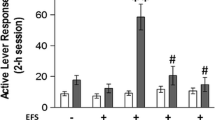
A dose of 1 mg/kg THC, but not 0.1–0.5 mg/kg, produced a conditioned place aversion (CPA) that was not modified by footshock delivered 24 h prior to conditioning trials; however, footshock delivered immediately prior to conditioning trials prevented that CPA. Lower doses of THC and URB597 produced no place conditioning regardless of footshock conditions. A dose of 1 mg/kg THC produced locomotor suppression during conditioning trials that was prevented by footshock delivered 24 h before and reversed to locomotor activation by footshock delivered immediately before conditioning.
Conclusions
Unlike the effect of footshock on THC- and URB597-induced anxiolytic effects, footshock does not promote THC or URB597-induced reward in a conditioned place preference paradigm. However, footshock stress reverses the sedative effects of 1 mg/kg THC.






Similar content being viewed by others
References
Amit Z, Corcoran ME, Charness ME, Shizgal P (1973) Intake of diazepam and hashish by alcohol preferring rats deprived of alcohol. Physiol Behav 10:523–527. doi:10.1016/0031-9384(73)90215-1
Bedse G, Hartly ND, Neale E et al (2017) Functional redundancy between canonical endocannabinoid signaling systems in the modulation of anxiety. Biol Psychiatry. doi:10.1016/j.biopsych.2017.03.002
Bluett RJ, Gamble-George JC, Hermanson DJ et al (2014) Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl Psychiatry. doi:10.1038/tp.2014.53
Bluett RJ, Baldi R, Haymer A et al (2017) Endocannabinoid signalling modulates susceptibility to traumatic stress exposure. Nat Commun. doi:10.1038/ncomms14782
Braida D, Iosuè S, Pegorini S, Sala M (2004) Δ 9-Tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharmacol 506:63–69. doi:10.1016/j.ejphar.2004.10.043
Chaperon F, Soubrie P, Puech AJ, Thiebot MH (1998) Involvement of central cannabinoid (CB1) receptors in the establishment of place conditioning in rats. Psychopharmacology 135(4):324–332. doi:10.1007/s002130050518
Cheer JF, Kendall DA, Marsden CA (2000) Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology 151:25–30. doi:10.1007/s002130000481
Cheer JF, Wassum KM, Heien MLAVM et al (2004) Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci 24:4393–4400. doi:10.1523/JNEUROSCI.0529-04.2004
Corcoran ME, Amit Z (1974) Reluctance of rats to drink hashish suspensions: free-choice and forced consumption, and the effects of hypothalamic stimulation. Psychopharmacologia 35:129–147. doi:10.1007/BF00429580
Cravatt BF, Giang DK, Mayfield SP et al (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87. doi:10.1038/384083a0
Cunningham C, Ferree N, Howard M (2003) Apparatus bias and place conditioning with ethanol in mice. Psychopharmachology 170(4):409–422. doi:10.1007/s00213-003-1559-y
De Luca MA, Valentini V, Bimpisidis Z et al (2014) Endocannabinoid 2-arachidonoylglycerol self-administration by Sprague-Dawley rats and stimulation of in vivo dopamine transmission in the nucleus accumbens shell. Front Psychiatry 5:1–9. doi:10.3389/fpsyt.2014.00140
De Luca MA, Bimpisidis Z, Melis M et al (2015) Stimulation of in vivo dopamine transmission and intravenous self-administration in rats and mice by JWH-018, a Spice cannabinoid. Neuropharmacology 99:705–714. doi:10.1016/j.neuropharm.2015.08.041
Devane WA, Hanuš L, Breuer A, Pertween RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258(5090):1946–1949
Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34:605–613
Dinh TP, Kathuria S, Piomelli D (2004) RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol 66:1260–1264. doi:10.1124/mol.104.002071
Fattore L, Cossu G, Martellotta CM, Fratta W (2001) Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology 156:410–416. doi:10.1007/s002130100734
Fegley D, Gaetani S, Duranti A et al (2005) Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 313:352–358. doi:10.1124/jpet.104.078980
Fokos S, Panagis G (2010) Effects of delta9-tetrahydrocannabinol on reward and anxiety in rats exposed to chronic unpredictable stress. J Psychopharmacol 24(5):767–777. doi:10.1177/0269881109104904
Ghozland S, Matthes HW, Simonin F et al (2002) Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci 22:1146–1154
Gobbi G, Bambico FR, Mangieri R et al (2005) Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A 102:18620–18625. doi:10.1073/pnas.0509591102
Green B, Kavanagh D, Young R (2003) Being stoned: a review of self-reported cannabis effects. Drug Alcohol Rev 22:453–460. doi:10.1080/09595230310001613976
Gregg J, Small E, Moore R et al (1976) Emotional response to intravenous delta9tetrahydrocannabinol during oral surgery. J Oral Surg (Chic) 34:301–331
Haller J, Barna I, Barsvari B et al (2009) Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmachologygy 204:607–616. doi:10.1007/s00213-009-1494-7
Haller J, Goldberg SR, Pelczer KG et al (2013) The effects of anandamide signaling enhanced by the FAAH inhibitor URB597 on coping styles in rats. Psychopharmacology 230:353–362. doi:10.1007/s00213-013-3161-2
Haller J, Aliczki M, Pelczer KG et al (2014) Effects of the fatty acid amide hydrolase inhibitor URB597 on coping behavior under challenging conditions in mice. Psychopharmacology 231:593–601. doi:10.1007/s00213-013-3273-8
Harris RT, Waters W, McLendon D (1974) Evaluation of reinforcing capability of delta-9-tetrahydrocannabinol in rhesus monkeys. Psychopharmacologia 37:23–29. doi:10.1007/BF00426679
Hempel BJ, Wakeford AGP, Clasen MM et al (2016) Delta-9-tetrahydrocannabinol (THC) history fails to affect THC’s ability to induce place preferences in rats. Pharmacol Biochem Behav 144:1–6. doi:10.1016/j.pbb.2016.02.007
Herman JP, Stinus L, Le Moal M (1984) Repeated stress increases locomotor response to amphetamine. Psychopharmacology 84:431–435. doi:10.1007/BF00555227
Hill MN, Gorzalka BB (2004) Enhancement of anxiety-like responsiveness to the cannabinoid CB 1 receptor agonist HU-210 following chronic stress. Eur J Pharmacol 499:291–295. doi:10.1016/j.ejphar.2004.06.069
Hutcheson DM, Tzavara ET, Smadja C et al (1998) Behavioural and biochemical evidence for signs of abstinence in mice chronically treated with delta-9-tetrahydrocannabinol. Br J Pharmacol 125:1567–1577. doi:10.1038/sj.bjp.0702228
Justinova Z, Tanda G, Redhi GH, Goldberg SR (2003) Self-administration of D9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychophamachology 169(2):135–140. doi:10.1007/s00213-003-1484-0
Kathuria S, Gaetani S, Fegley D et al (2003) Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 9:76–81. doi:10.1038/nm803
Le Foll B, Wiggins M, Goldberg SR (2006) Nicotine pre-exposure does not potentiate the locomotor or rewarding effects of Delta-9-tetrahydrocannabinol in rats. Behav Pharmacol 17:195–199. doi:10.1097/01.fbp.0000197460.16516.81
Lecca D, Cacciapaglia F, Valentini V, Di Chiara G (2006) Monitoring extracellular dopamine in the rat nucleus accumbens shell and core during acquisition and maintenance of intravenous WIN 55,212-2 self-administration. Psychopharmacology 188:63–74. doi:10.1007/s00213-006-0475-3
Leite JR, Carlini E (1974) Failure to obtain “cannabis-directed behavior” and abstinence syndrome in rats chronically treated with Cannabis sativa extracts. Psychopharmacologia 36:133–145
Lepore M, Vorel SR, Lowinson J, Gardner EL (1995) Conditioned place preference induced by delta9-tetrahydrocannabinol: comparison with cocain, morphine, and food reward. Life Sci 56:2073–2080
Leyton M, Stewart J (1990) Preexposure to foot-shock sensitizes the locomotor response to subsequent systemic morphine and intra-nucleus accumbens amphetamine. Pharmacol Biochem Behav 37:303–310. doi:10.1016/0091-3057(90)90339-J
Li J-X, Koek W, France CP (2012) Interactions between Δ(9)-tetrahydrocannabinol and heroin: self-administration in rhesus monkeys. Behav Pharmacol 23:754–761. doi:10.1097/FBP.0b013e32835a3907
Mallet PE, Beninger RJ (1998) Delta-9-tetrahydrocannabinol, but not the endogenous ligand anandamide, produces conditioned place avoidance. Life Sci 62:2431–2439
Martellotta MC, Cossu G, Fattore L et al (1998) Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience 85:327–330. doi:10.1016/S0306-4522(98)00052-9
McGregor IS, Issakidis CN, Prior G (1996) Aversive effects of the synthetic cannabinoid CP 55,940 in rats. Pharmacol Biochem Behav 53:657–664
Mechoulam R, Gaoni Y (1965) A total synthesis of Dl-delta-1-tetrahydrocannabinol, the active constituent of hashish. J Am Chem Soc 87:3273–3275
Mechoulam R, Ben-Shabat S, Hanus L et al (1995) Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90. doi:10.1016/0006-2952(95)00109-D
Mechoulam R, Parker LA (2013) The endocannabinoid system and the brain. Annu Rev Psychol 64:21–47. doi:10.1146/annurev-psych-113011-143739
Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH (2007) Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmachology 192(1):61–70. doi:10.1007/s00213-006-0689-4
Naliboff B, Rickles W, Cohen M, Naimark R (1976) Interactions of MArijuana and induced stess: forearm blood flow, heart rate, and skin conductance. Psychophysiology 13:517–522
Parker LA, Gillies T (1995) THC-induced place and taste aversions in Lewis and Sprague-Dawley rats. Behav Neurosci 109:71–78
Parker LA, Limebeer CL, Simpson GR (1998) Chlordiazepoxide-induced conditioned place and taste aversion learning in rats. Pharmacol Biochem Behav 59:33–37. doi:10.1016/S0091-3057(97)00333-X
Parker LA, Burton P, Sorge RE et al (2004) Effect of low doses of delta9-tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats. Psychopharmacology 175:360–366. doi:10.1007/s00213-004-1825-7
Patel S, Roelke CT, Rademacher DJ, Hillard CJ (2005) Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci 21:1057–1069. doi:10.1111/j.1460-9568.2005.03916.x
Patel S, Hill MN, Hillard CJ (2014) Effects of phytocannabinoids on anxiety, mood, and the endocrine system. In: Pertween RG (ed) Handbook of cannabis. Oxford University Press, Oxford
Pickens R, Thompson T, Muchow DC (1973) Cannabis and phencyclidine self-administration by animals. Psych Depend 78–86
Quinn HR, Matsumoto I, Callaghan PD et al (2008) Adolescent rats find repeated D 9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychologia 33:1113–1126. doi:10.1038/sj.npp.1301475
Reilly D, Didcott P, Ift WESW (1998) Long-term cannabis use: characteristics of users in an Australian rural area. Addiction 93:837–846
Rock EM, Limebeer CL, Petrie GN et al (2017) Effect of prior foot shock stress and Δ9-tetrahydrocannabinol, cannabidiolic acid, and cannabidiol on anxiety-like responding in the light-dark emergence test in rats. Psychopharmacology:1–11. doi:10.1007/s00213-017-4626-5
Sanudo-Pena M, Tsou K, Delay E et al (1997) Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci Lett 223:125–128
Soria G, Castañé A, Berrendero F et al (2004) Adenosine A2A receptors are involved in physical dependence and place conditioning induced by THC. Eur J Neurosci 20:2203–2213. doi:10.1111/j.1460-9568.2004.03682.x
Speciale SG, Miller JD, McMillen BA, German DC (1986) Activation of specific central dopamine pathways: locomotion and footshock. Brain Res Bull 16:33–38. doi:10.1016/0361-9230(86)90009-2
Tanda G, Pontieri F, Chiara G (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common u1 opioid receptor mechanism. Am Assoc forthe Adv Sci 276:2048–2050
Tanda G, Munzar P, Goldberg SR (2000) Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci 3:1073–1074. doi:10.1038/80577
Tanda G (2016) Preclinical studies on the reinforcing effects of cannabinoids. A tribute to the scientific research of Dr. Steve Goldberg. Psychopharmacology 233:1845–1866. doi:10.1007/s00213-016-4244-7
Tzschentke TM (2007) Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462. doi:10.1111/j.1369-1600.2007.00070.x
Valjent E, Maldonado R (2000) A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology 147:436–438. doi:10.1007/s002130050013
Vann RE, Gamage TF, Warner JA et al (2008) Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of delta9-tetrahydrocannabinol. Drug Alcohol Depend 94:191–198. doi:10.1016/j.drugalcdep.2007.11.017
Vlachou S, Nomikos GG, Stephens DN, Panagis G (2007) Lack of evidence for appetitive effects of delta 9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav Pharmacol 18:311–319. doi:10.1097/FBP.0b013e3282186cf2
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492. doi:10.1037/0033-295X.94.4.469
Zangen A, Solinas M, Ikemoto S et al (2006) Two brain sites for cannabinoid reward. J Neurosci 26:4901–4907
Acknowledgments
The idea for the stress manipulation arose from a question asked by Dr. Aron Lichtman during the International Cannabinoid Research Society Meetings, Bukovina, Poland, 2016. This research was supported by grants from the Natural Science and Engineering Research Council (92057) and the Canadian Institutes or Health Reaserch (137122) to L. Parker.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal procedures were approved by the Animal Care Committee of the University of Guelph and adhere to the guidelines of the Canadian Council of Animal Care.
Rights and permissions
About this article
Cite this article
DeVuono, M.V., Wills, K.L., MacPherson, D.V. et al. Effect of footshock stress on place conditioning produced by Δ9-tetrahydrocannabinol and the fatty acid amide hydrolase (FAAH) inhibitor, URB597, in Sprague-Dawley rats. Psychopharmacology 234, 3229–3240 (2017). https://doi.org/10.1007/s00213-017-4714-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4714-6