Abstract
Rationale
Nicotine dependence is characterized as a neural circuit dysfunction, particularly with regard to the reward circuit. Although dependence severity moderates cue reactivity in the brain regions involved in reward processing, the direction of its influence remains controversial.
Objectives
Investigating the functional organization of the reward circuit may provide complementary information. Here, we used resting-state functional connectivity (rsFC) to evaluate the integrity of the reward circuit in smokers with different severities of nicotine dependence.
Methods
Totals of 65 smokers and 37 non-smokers underwent resting-state functional magnetic resonance imaging (fMRI). The smokers were divided into low-dependent (FTND < 5, n = 26) and high-dependent smoker groups (FTND ≥ 5, n = 39) based on their nicotine-dependence severity (as measured by the Fagerström test for nicotine dependence [FTND]). The region of interest (ROI)-wise rsFC within the reward circuit was compared between smokers and non-smokers as well as between low-dependent and high-dependent smokers and then correlated with smokers’ FTND scores.
Results
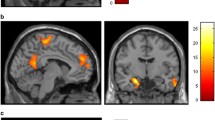
Widespread rsFC attenuation was observed in the reward circuit of smokers compared with non-smokers. Compared with low-dependent smokers, high-dependent smokers showed greater rsFC between the right amygdala and the left nucleus accumbens (NAcc) as well as between the bilateral hippocampus. Furthermore, a positive correlation between the inter-hippocampus rsFC and the severity of nicotine dependence (FTND) was detected among all smokers (r = 0.416, p = 0.001).
Conclusions
Our results indicate a dysfunction of the reward circuit in nicotine-dependent individuals. Moreover, our study improves the understanding of the neuroplastic changes that occur during the development of nicotine dependence.



Similar content being viewed by others
References
Adinoff B, Gu H, Merrick C, McHugh M, Jeon-Slaughter H, Lu H, Yang Y, Stein EA (2015) Basal hippocampal activity and its functional connectivity predicts cocaine relapse. Biol Psychiatry 78:496–504
Baker A, Richmond R, Haile M, Lewin TJ, Carr VJ, Taylor RL, Constable PM, Jansons S, Wilhelm K, Moeller-Saxone K (2007) Characteristics of smokers with a psychotic disorder and implications for smoking interventions. Psychiatry Res 150:141–152
Bi Y, Yuan K, Guan Y, Cheng J, Zhang Y, Li Y, Yu D, Qin W, Tian J (2016) Altered resting state functional connectivity of anterior insula in young smokers. Brain Imaging Behav. doi:10.1007/s11682-016-9511-z
Breslau N, Kilbey M, Andreski P (1991) Nicotine dependence, major depression, and anxiety in young adults. Arch Gen Psychiatry 48:1069–1074
Breslau N, Kilbey MM, Andreski P (1993) Nicotine dependence and major depression. New evidence from a prospective investigation. Arch Gen Psychiatr 50:31–35
Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED (2004) Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry 55:77–84
Buhler M, Vollstadt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, Buchel C, Smolka MN (2010) Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biol Psychiatry 67:745–752
Caponnetto P, Polosa R (2008) Common predictors of smoking cessation in clinical practice. Respir Med 102:1182–1192
Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE (2013) Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 38:2363–2372
Clewett D, Luo S, Hsu E, Ainslie G, Mather M, Monterosso J (2014) Increased functional coupling between the left fronto-parietal network and anterior insula predicts steeper delay discounting in smokers. Hum Brain Mapp 35:3774–3787
Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD (2010) Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. NeuroImage 52:590–599
David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT (2005) Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry 58:488–494
Di Ciano P, Everitt BJ (2004) Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci: Off J Soc Neurosci 24:7167–7173
Ding X, Lee SW (2013) Changes of functional and effective connectivity in smoking replenishment on deprived heavy smokers: a resting-state FMRI study. PLoS One 8:e59331
Due DL, Huettel SA, Hall WG, Rubin DC (2002) Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry 159:954–960
Duriez Q, Crivello F, Mazoyer B (2014) Sex-related and tissue-specific effects of tobacco smoking on brain atrophy: assessment in a large longitudinal cohort of healthy elderly. Front Aging Neurosci 6:299
Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489
Fagerstrom K, Russ C, Yu CR, Yunis C, Foulds J (2012) The Fagerstrom test for nicotine dependence as a predictor of smoking abstinence: a pooled analysis of varenicline clinical trial data. Nicotine Tob Res: Off J Soc Res Nicotine Tob 14:1467–1473
Feltenstein MW, See RE (2008) The neurocircuitry of addiction: an overview. Br J Pharmacol 154:261–274
Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'Brien CP, Detre JA, Childress AR (2007) Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 32:2301–2309
Franklin TR, Wetherill RR, Jagannathan K, Johnson B, Mumma J, Hager N, Rao H, Childress AR (2014) The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLoS One 9:e104102
Fritz HC, Wittfeld K, Schmidt CO, Domin M, Grabe HJ, Hegenscheid K, Hosten N, Lotze M (2014) Current smoking and reduced gray matter volume—a voxel-based morphometry study. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 39:2594–2600
Froeliger B, Kozink RV, Rose JE, Behm FM, Salley AN, McClernon FJ (2010) Hippocampal and striatal gray matter volume are associated with a smoking cessation treatment outcome: results of an exploratory voxel-based morphometric analysis. Psychopharmacology 210:577–583
Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M (2006) Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci 24:1744–1750
Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652
Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA (1996) Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature 383:713–716
Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y (2010) Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage 53:593–601
Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 35:4–26
Hanlon CA, Owens MM, Joseph JE, Zhu X, George MS, Brady KT, Hartwell KJ (2014) Lower subcortical gray matter volume in both younger smokers and established smokers relative to non-smokers. Addict Biol
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict 86:1119–1127
Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA (2009) Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry 66:431–441
Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, Salmeron BJ, Srivastava V, Thaker GK, Goldman D, Stein EA (2010) A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci U S A 107:13509–13514
Huang W, DiFranza JR, Kennedy DN, Zhang N, Ziedonis D, Ursprung S, King JA (2013) Progressive levels of physical dependence to tobacco coincide with changes in the anterior cingulum bundle microstructure. PLoS One 8:e67837
Huang W, King JA, Ursprung WW, Zheng S, Zhang N, Kennedy DN, Ziedonis D, DiFranza JR (2014) The development and expression of physical nicotine dependence corresponds to structural and functional alterations in the anterior cingulate-precuneus pathway. Brain and behavior 4:408–417
Jackson ME, Moghaddam B (2001) Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex. J Neurosci: Off J Soc Neurosci 21:676–681
Janes AC, Nickerson LD, Frederick Bde B, Kaufman MJ (2012a) Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend 125:252–259
Janes AC, Smoller JW, David SP, Frederick BD, Haddad S, Basu A, Fava M, Evins AE, Kaufman MJ (2012b) Association between CHRNA5 genetic variation at rs16969968 and brain reactivity to smoking images in nicotine dependent women. Drug Alcohol Depend 120:7–13
Japuntich SJ, Leventhal AM, Piper ME, Bolt DM, Roberts LJ, Fiore MC, Baker TB (2011) Smoker characteristics and smoking-cessation milestones. Am J Prev Med 40:286–294
Jasinska AJ, Zorick T, Brody AL, Stein EA (2014) Dual role of nicotine in addiction and cognition: a review of neuroimaging studies in humans. Neuropharmacology 84:111–122
Keller J, Young CB, Kelley E, Prater K, Levitin DJ, Menon V (2013) Trait anhedonia is associated with reduced reactivity and connectivity of mesolimbic and paralimbic reward pathways. J Psychiatr Res 47:1319–1328
Kenney JW, Gould TJ (2008) Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol 38:101–121
Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ (2013) Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA Psychiatry 70:857–868
Li Y, Yuan K, Cai C, Feng D, Yin J, Bi Y, Shi S, Yu D, Jin C, von Deneen KM, Qin W, Tian J (2015) Reduced frontal cortical thickness and increased caudate volume within fronto-striatal circuits in young adult smokers. Drug Alcohol Depend 151:211–219
Liao Y, Tang J, Liu T, Chen X, Hao W (2012) Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol 17:977–980
Lin F, Zhou Y, Du Y, Zhao Z, Qin L, Xu J, Lei H (2015) Aberrant corticostriatal functional circuits in adolescents with Internet addiction disorder. Front Hum Neurosci 9:356
Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR (2010) Addiction related alteration in resting-state brain connectivity. NeuroImage 49:738–744
Markou A (2008) Review. Neurobiology of nicotine dependence. Philos Trans R Soc Lond Ser B Biol Sci 363:3159–3168
McClernon FJ, Kozink RV, Rose JE (2008) Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 33:2148–2157
Mendrek A, Dinh-Williams L, Bourque J, Potvin S (2014) Sex differences and menstrual cycle phase-dependent modulation of craving for cigarette: an FMRI pilot study. Psychiatry J 2014:723632
Pan P, Shi H, Zhong J, Xiao P, Shen Y, Wu L, Song Y, He G (2013) Chronic smoking and brain gray matter changes: evidence from meta-analysis of voxel-based morphometry studies. Neurol Sci: Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 34:813–817
Pistillo F, Fasoli F, Moretti M, McClure-Begley T, Zoli M, Marks MJ, Gotti C (2015) Chronic nicotine and withdrawal affect glutamatergic but not nicotinic receptor expression in the mesocorticolimbic pathway in a region-specific manner. Pharmacol Res 103:167–176
Ryan KK, Mackillop J, Carpenter MJ (2013) The relationship between impulsivity, risk-taking propensity and nicotine dependence among older adolescent smokers. Addict Behav 38:1431–1434
Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF (2006) Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology 184:577–588
Stewart J (2004) Pathways to relapse: factors controlling the reinitiation of drug seeking after abstinence. Neb Symp Motiv Neb Symp Motiv 50:197–234
Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475:377–380
Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA (2013) Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry 74:538–546
Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, Woicik PA, Telang F, Goldstein RZ (2010) Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One 5:e10815
Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F (2011) Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A 108:15037–15042
Volkow ND, Wang GJ, Tomasi D, Baler RD (2013) Unbalanced neuronal circuits in addiction. Curr Opin Neurobiol 23:639–648
Vollstadt-Klein S, Kobiella A, Buhler M, Graf C, Fehr C, Mann K, Smolka MN (2011) Severity of dependence modulates smokers’ neuronal cue reactivity and cigarette craving elicited by tobacco advertisement. Addict Biol 16:166–175
Wang L, Negreira A, LaViolette P, Bakkour A, Sperling RA, Dickerson BC (2010) Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus 20:345–351
Wellman RJ, DiFranza JR, O'Loughlin J (2014) Recalled first reactions to inhaling nicotine predict the level of physical dependence. Drug Alcohol Depend 143:167–172
Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR (2011) Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend 115:137–144
Xie C, Shao Y, Ma L, Zhai T, Ye E, Fu L, Bi G, Chen G, Cohen A, Li W, Chen G, Yang Z, Li SJ (2014) Imbalanced functional link between valuation networks in abstinent heroin-dependent subjects. Mol Psychiatry 19:10–12
Yan C, Liu D, He Y, Zou Q, Zhu C, Zuo X, Long X, Zang Y (2009) Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PLoS One 4:e5743
Zhai T, Shao Y, Chen G, Ye E, Ma L, Wang L, Lei Y, Chen G, Li W, Zou F, Jin X, Li SJ, Yang Z (2015) Nature of functional links in valuation networks differentiates impulsive behaviors between abstinent heroin-dependent subjects and nondrug-using subjects. NeuroImage 115:76–84
Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA (2011a) Factors underlying prefrontal and insula structural alterations in smokers. NeuroImage 54:42–48
Zhang X, Salmeron BJ, Ross TJ, Gu H, Geng X, Yang Y, Stein EA (2011b) Anatomical differences and network characteristics underlying smoking cue reactivity. NeuroImage 54:131–141
Acknowledgments
Grants from the National Natural Science Foundation of China (No. 81171310) and the Scientific Research Project of Zhejiang Province (2011C23094) supported this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Institutional Review Board of the Second Affiliated Hospital of the Zhejiang University School of Medicine reviewed and approved all procedures. All participants provided signed informed consents prior to study participation.
Conflicts of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Shen, Z., Huang, P., Qian, W. et al. Severity of dependence modulates smokers’ functional connectivity in the reward circuit: a preliminary study. Psychopharmacology 233, 2129–2137 (2016). https://doi.org/10.1007/s00213-016-4262-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4262-5