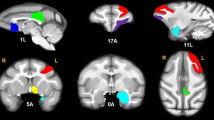
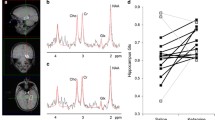
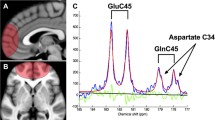
Abstract
Rationale
Aberrant prefrontal-hippocampal (PFC-HC) connectivity is disrupted in several psychiatric and at-risk conditions. Advances in rodent functional imaging have opened the possibility that this phenotype could serve as a translational imaging marker for psychiatric research. Recent evidence from functional magnetic resonance imaging (fMRI) studies has indicated an increase in PFC-HC coupling during working-memory tasks in both schizophrenic patients and at-risk populations, in contrast to a decrease in resting-state PFC-HC connectivity. Acute ketamine challenge is widely used in both humans and rats as a pharmacological model to study the mechanisms of N-methyl-d-aspartate (NMDA) receptor hypofunction in the context of psychiatric disorders.
Objectives
We aimed to establish whether acute ketamine challenge has consistent effects in rats and humans by investigating resting-state fMRI PFC-HC connectivity and thus to corroborate its potential utility as a translational probe.
Methods
Twenty-four healthy human subjects (12 females, mean age 25 years) received intravenous doses of either saline (placebo) or ketamine (0.5 mg/kg body weight). Eighteen Sprague-Dawley male rats received either saline or ketamine (25 mg/kg). Resting-state fMRI measurements took place after injections, and the data were analyzed for PFC-HC functional connectivity.
Results
In both species, ketamine induced a robust increase in PFC-HC coupling, in contrast to findings in chronic schizophrenia.
Conclusions
This translational comparison demonstrates a cross-species consistency in pharmacological effect and elucidates ketamine-induced alterations in PFC-HC coupling, a phenotype often disrupted in pathological conditions, which may give clue to understanding of psychiatric disorders and their onset, and help in the development of new treatments.




Similar content being viewed by others
References
Becerra L, Upadhyay J, Chang PC, Bishop J, Anderson J, Baumgartner R, Schwarz AJ, Coimbra A, Wallin D, Nutile L, George E, Maier G, Sunkaraneni S, Iyengar S, Evelhoch JL, Bleakman D, Hargreaves R, Borsook D (2013) Parallel buprenorphine phMRI responses in conscious rodents and healthy human subjects. J Pharmacol Exp Ther 345:41–51. doi:10.1124/jpet.112.201145
Behzadi Y, Restom K, Liau J, Liu TT (2007) A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37:90–101
Burdett NG, Menon DK, Carpenter TA, Jones JG, Hall LD (1995) Visualisation of changes in regional cerebral blood flow (rCBF) produced by ketamine using long TE gradient-echo sequences: preliminary results. Magn Reson Imaging 13:549–553
Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, Weinberger DR (2003) Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry 160:709–719
Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S (2014) Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J Cogn Neurosci 26:501–513. doi:10.1162/jocn_a_00517
Chudasama Y (2011) Animal models of prefrontal-executive function. Behav Neurosci 125:327–343. doi:10.1037/a0023766
Corlett PR, Honey GD, Krystal JH, Fletcher PC (2011) Glutamatergic model psychoses: prediction error, learning, and inference. Neuropsychopharmacology 36:294–315. doi:10.1038/npp.2010.163
Crosby G, Crane AM, Sokoloff L (1982) Local changes in cerebral glucose utilization during ketamine anesthesia. Anesthesiology 56:437–443
D’Souza DC, Singh N, Elander J, Carbuto M, Pittman B, Udo de Haes J, Sjogren M, Peeters P, Ranganathan M, Schipper J (2012) Glycine transporter inhibitor attenuates the psychotomimetic effects of ketamine in healthy males: preliminary evidence. Neuropsychopharmacology 37:1036–1046. doi:10.1038/npp.2011.295
Dawson N, Morris BJ, Pratt JA (2013) Subanaesthetic ketamine treatment alters prefrontal cortex connectivity with thalamus and ascending subcortical systems. Schizophr Bull 39:366–377. doi:10.1093/schbul/sbr144; 10.1093/schbul/sbr144
Dawson N, McDonald M, Higham DJ, Morris BJ, Pratt JA (2014) Subanesthetic ketamine treatment promotes abnormal interactions between neural subsystems and alters the properties of functional brain networks. Neuropsychopharmacology 39:1786–1798. doi:10.1038/npp.2014.26
Diamond PR, Farmery AD, Atkinson S, Haldar J, Williams N, Cowen PJ, Geddes JR, McShane R (2014) Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J Psychopharmacol 28:536–544
Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, Gueorguieva R, He G, Ramachandran R, Suckow RF, Anticevic A, Morgan PT, Krystal JH (2013) Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. doi:10.1038/mp.2012.194
Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, Haddad L, Mier D, Opitz von Boberfeld C, Raab K, Witt SH, Rietschel M, Cichon S, Meyer-Lindenberg A (2009) Neural mechanisms of a genome-wide supported psychosis variant. Science 324:605. doi:10.1126/science.1167768
Floresco SB, Todd CL, Grace AA (2001) Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci 21:4915–4922
Furey ML, Khanna A, Hoffman EM, Drevets WC (2010) Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacology 35:2479–2488. doi:10.1038/npp.2010.131
Garcia LS, Comim CM, Valvassori SS, Reus GZ, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008) Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels. Basic Clin Pharmacol Toxicol 103:502–506. doi:10.1111/j.1742-7843.2008.00210.x
Gass N, Schwarz AJ, Sartorius A, Schenker E, Risterucci C, Spedding M, Zheng L, Meyer-Lindenberg A, Weber-Fahr W (2014) Sub-anesthetic ketamine modulates intrinsic BOLD connectivity within the hippocampal-prefrontal circuit in the rat. Neuropsychopharmacology 39:895–906. doi:10.1038/npp.2013.290
Glick SD, Ross DA (1981) Lateralization of function in the rat brain. Trends Neurosci. 4:196–199
Godsil BP, Kiss JP, Spedding M, Jay TM (2013) The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol. doi:10.1016/j.euroneuro.2012.10.018
Grandjean J, Schroeter A, Batata I, Rudin M (2014) Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage 102(Pt 2):838–847. doi:10.1016/j.neuroimage.2014.08.043
Harrison PJ, Law AJ, Eastwood SL (2003) Glutamate receptors and transporters in the hippocampus in schizophrenia. Ann N Y Acad Sci 1003:94–101
Heckers S, Konradi C (2014) GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. doi:10.1016/j.schres.2014.09.041
Hong LE, Summerfelt A, Buchanan RW, O’Donnell P, Thaker GK, Weiler MA, Lahti AC (2010) Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology 35:632–640. doi:10.1038/npp.2009.168
Igloi K, Doeller CF, Berthoz A, Rondi-Reig L, Burgess N (2010) Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Natl Acad Sci U S A 107:14466–14471. doi:10.1073/pnas.1004243107
Joules R, Doyle OM, Schwarz AJ, O’Daly OG, Brammer M, Williams SC, Mehta MA (2015) Ketamine induces a robust whole-brain connectivity pattern that can be differentially modulated by drugs of different mechanism and clinical profile. Psychopharmacology (Berl). doi:10.1007/s00213-015-3951-9
Jung MW, Qin Y, McNaughton BL, Barnes CA (1998) Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex 8:437–450
Kerwin RW, Patel S, Meldrum BS, Czudek C, Reynolds GP (1988) Asymmetrical loss of glutamate receptor subtype in left hippocampus in schizophrenia. Lancet 1:583–584
Kesner RP, Churchwell JC (2011) An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem 96:417–431. doi:10.1016/j.nlm.2011.07.002
Khalili-Mahani N, Niesters M, van Osch MJ, Oitzl M, Veer I, de Rooij M, van Gerven J, van Buchem MA, Beckmann CF, Rombouts SA, Dahan A (2015) Ketamine interactions with biomarkers of stress: a randomized placebo-controlled repeated measures resting-state fMRI and PCASL pilot study in healthy men. Neuroimage 108:396–409. doi:10.1016/j.neuroimage.2014.12.050
Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B (2012) Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Struct Funct 217:395–409. doi:10.1007/s00429-011-0351-8
Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S (2011) Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res 131:165–173. doi:10.1016/j.schres.2011.06.007
Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A (2005) Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 179:303–309. doi:10.1007/s00213-004-1982-8
Liang Z, King J, Zhang N (2012) Intrinsic organization of the anesthetized brain. J Neurosci 32:10191. doi:10.1523/JNEUROSCI.1020-12.2012
Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T (2008) Disrupted small-world networks in schizophrenia. Brain 131:945–961. doi:10.1093/brain/awn018
Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA (2003) Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience 117:697–706
Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E (2010) Functional connectivity and brain networks in schizophrenia. J Neurosci 30:9477–9487. doi:10.1523/JNEUROSCI.0333-10.2010
Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF (2001) Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 158:1809–1817
Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF (2005) Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62:379–386. doi:10.1001/archpsyc.62.4.379
Micheloyannis S, Pachou E, Stam CJ, Breakspear M, Bitsios P, Vourkas M, Erimaki S, Zervakis M (2006) Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res 87:60–66
Mion G, Villevieille T (2013) Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther 19:370–380. doi:10.1111/cns.12099
Moghaddam B, Adams B, Verma A, Daly D (1997) Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17:2921–2927
Moran RJ, Jones MW, Blockeel AJ, Adams RA, Stephan KE, Friston KJ (2015) Losing control under ketamine: suppressed cortico-hippocampal drive following acute ketamine in rats. Neuropsychopharmacology 40:268–277. doi:10.1038/npp.2014.184
Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV (2004) Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose-response study. Psychopharmacology (Berl) 172:298–308. doi:10.1007/s00213-003-1656-y
Nelson SR, Howard RB, Cross RS, Samson F (1980) Ketamine-induced changes in regional glucose utilization in the rat brain. Anesthesiology 52:330–334
Niciu MJ, Luckenbaugh DA, Ionescu DF, Richards EM, Vande Voort JL, Ballard ED, Brutsche NE, Furey ML, Zarate CA, Jr (2014) Ketamine’s antidepressant efficacy is extended for at least four weeks in subjects with a family history of an alcohol use disorder. Int J Neuropsychopharmacol 18:doi: 10.1093/ijnp/pyu039
Olney JW, Farber NB (1995) NMDA antagonists as neurotherapeutic drugs, psychotogens, neurotoxins, and research tools for studying schizophrenia. Neuropsychopharmacology 13:335–345. doi:10.1016/0893-133X(95)00079-S
Phillips KG, Cotel MC, McCarthy AP, Edgar DM, Tricklebank M, O’Neill MJ, Jones MW, Wafford KA (2012) Differential effects of NMDA antagonists on high frequency and gamma EEG oscillations in a neurodevelopmental model of schizophrenia. Neuropharmacology 62:1359–1370. doi:10.1016/j.neuropharm.2011.04.006
Pinault D (2008) N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry 63:730–735. doi:10.1016/j.biopsych.2007.10.006
Pitsikas N, Boultadakis A, Sakellaridis N (2008) Effects of sub-anesthetic doses of ketamine on rats’ spatial and non-spatial recognition memory. Neuroscience 154:454–460. doi:10.1016/j.neuroscience.2008.04.001
Preuss TM (1995) Do rats have prefrontal cortex? The rose-woolsey-akert program reconsidered. J Cogn Neurosci 7:1–24. doi:10.1162/jocn.1995.7.1.1
Qi H, Mailliet F, Spedding M, Rocher C, Zhang X, Delagrange P, McEwen B, Jay TM, Svenningsson P (2009) Antidepressants reverse the attenuation of the neurotrophic MEK/MAPK cascade in frontal cortex by elevated platform stress; reversal of effects on LTP is associated with GluA1 phosphorylation. Neuropharmacology 56:37–46. doi:10.1016/j.neuropharm.2008.06.068
Rasetti R, Sambataro F, Chen Q, Callicott JH, Mattay VS, Weinberger DR (2011) Altered cortical network dynamics: a potential intermediate phenotype for schizophrenia and association with ZNF804A. Arch Gen Psychiatry 68:1207–1217. doi:10.1001/archgenpsychiatry.2011.103
Rocher C, Spedding M, Munoz C, Jay TM (2004) Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex 14:224–229
Rogers LJ (1989) Laterality in animals. Int J Comp Psychol 3(1):5–25
Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE (2010) Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res 117:21–30. doi:10.1016/j.schres.2010.01.001
Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, Barrow R, Yeo R, Lauriello J, Brooks WM (2005) Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry 162:394–396
Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Holroyd T, DiazGranados N, Machado-Vieira R, Grillon C, Drevets WC, Zarate CA Jr (2010) Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology 35:1415–1422. doi:10.1038/npp.2010.24
Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H, Boesiger P, Henning A, Seifritz E (2012) Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One 7:e44799. doi:10.1371/journal.pone.0044799
Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, Small SA (2013) Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78:81–93. doi:10.1016/j.neuron.2013.02.011
Schwarz AJ, Danckaert A, Reese T, Gozzi A, Paxinos G, Watson C, Merlo-Pich EV, Bifone A (2006) A stereotaxic MRI template set for the rat brain with tissue class distribution maps and co-registered anatomical atlas: application to pharmacological MRI. Neuroimage 32:538–550
Schwarz AJ, Gass N, Sartorius A, Zheng L, Spedding M, Schenker E, Risterucci C, Meyer-Lindenberg A, Weber-Fahr W (2013) The low-frequency blood oxygenation level-dependent functional connectivity signature of the hippocampal-prefrontal network in the rat brain. Neuroscience 228:243–258. doi:10.1016/j. neuroscience .2012.10.032
Seamans JK, Lapish CC, Durstewitz D (2008) Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res 14:249–262. doi:10.1007/BF03033814
Seidman LJ, Thermenos HW, Poldrack RA, Peace NK, Koch JK, Faraone SV, Tsuang MT (2006) Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophr Res 85:58–72
Skoblenick K, Everling S (2012) NMDA antagonist ketamine reduces task selectivity in macaque dorsolateral prefrontal neurons and impairs performance of randomly interleaved prosaccades and antisaccades. J Neurosci 32:12018–12027. doi:10.1523/JNEUROSCI.1510-12.2012
Spedding M, Neau I, Harsing L (2003) Brain plasticity and pathology in psychiatric disease: sites of action for potential therapy. Curr Opin Pharmacol 3:33–40
Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ, Krystal JH, Nutt D, Barker GJ (2012) Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry 17:664–665. doi:10.1038/mp.2011.171
Strange BA, Witter MP, Lein ES, Moser EI (2014) Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 15:655–669. doi:10.1038/nrn3785
Suazo V, Diez A, Tamayo P, Montes C, Molina V (2013) Limbic hyperactivity associated to verbal memory deficit in schizophrenia. J Psychiatr Res 47:843–850. doi:10.1016/j.jpsychires.2013.02.007
Svenningsson P, Bateup H, Qi H, Takamiya K, Huganir RL, Spedding M, Roth BL, McEwen BS, Greengard P (2007) Involvement of AMPA receptor phosphorylation in antidepressant actions with special reference to tianeptine. Eur J Neurosci 26:3509–3517
Tagliazucchi E, von Wegner F, Morzelewski A, Brodbeck V, Laufs H (2012) Dynamic BOLD functional connectivity in humans and its electrophysiological correlates. Front Hum Neurosci 6:339. doi:10.3389/fnhum.2012.00339
Uylings HB, Groenewegen HJ, Kolb B (2003) Do rats have a prefrontal cortex? Behav Brain Res 146:3–17
van Buuren M, Gladwin TE, Zandbelt BB, van den Heuvel M, Ramsey NF, Kahn RS, Vink M (2009) Cardiorespiratory effects on default-mode network activity as measured with fMRI. Hum Brain Mapp 30:3031–3042. doi:10.1002/hbm.20729
Vollenweider FX, Kometer M (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11:642–651. doi:10.1038/nrn2884
Vollenweider FX, Leenders KL, Scharfetter C, Antonini A, Maguire P, Missimer J, Angst J (1997) Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG). Eur Neuropsychopharmacol 7:9–24
Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141. doi:10.1089/brain.2012.0073
Williams KA, Magnuson M, Majeed W, LaConte SM, Peltier SJ, Hu X, Keilholz SD (2010) Comparison of alpha-chloralose, medetomidine and isoflurane anesthesia for functional connectivity mapping in the rat. Magn Reson Imaging 28:995–1003. doi:10.1016/j.mri.2010.03.007
Wise SP (2008) Forward frontal fields: phylogeny and fundamental function. Trends Neurosci 31:599–608. doi:10.1016/j.tins.2008.08.008
Yang ST, Shi Y, Wang Q, Peng JY, Li BM (2014) Neuronal representation of working memory in the medial prefrontal cortex of rats. Mol Brain 7:61. doi:10.1186/s13041-014-0061-2
Zarate CA Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, Wainer IW (2012) Relationship of ketamine's plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry 72:331–338. doi:10.1016/j.biopsych.2012.03.004
Zarate CA Jr, Mathews DC, Furey ML (2013) Human biomarkers of rapid antidepressant effects. Biol Psychiatry 73:1142–1155. doi:10.1016/j.biopsych.2012.11.031
Zeilhofer HU, Swandulla D, Geisslinger G, Brune K (1992) Differential effects of ketamine enantiomers on NMDA receptor currents in cultured neurons. Eur J Pharmacol 213:155–158
Zhang H, Etherington LA, Hafner AS, Belelli D, Coussen F, Delagrange P, Chaouloff F, Spedding M, Lambert JJ, Choquet D, Groc L (2013) Regulation of AMPA receptor surface trafficking and synaptic plasticity by a cognitive enhancer and antidepressant molecule. Mol Psychiatry 18:471–484. doi:10.1038/mp.2012.80
Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H, Yu C, Liu Z, Jiang T (2008) Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res 100:120–132. doi:10.1016/j.schres.2007.11.039
Acknowledgments
The authors thank Felix Hörner and Claudia Falfan-Melgoza for their excellent technical assistance.
Conflict of interest
NEWMEDS—the research leading to these results, has received support from the Innovative Medicine Initiative Joint Undertaking under Grant Agreement No. 115008 of which resources are composed of the European Federation of Pharmaceutical Industries and Associations (EFPIA) in-kind contribution and financial contribution from the European Union’s Seventh Framework Program (FP7/2007–2013). Also the work was supported by the BMBF (01EW1110) in the frame of ERA-Net NEURON. AJS is an employee and shareholder of Eli Lilly and Company; ES is an employee of Instituts de Recherches Servier; MS is an employee of Spedding Research Solutions; and CR is an employee of F. Hoffman-La Roche.
Author information
Authors and Affiliations
Corresponding author
Additional information
Oliver Grimm and Natalia Gass shared first authorship.
Adam J. Schwarz and Andreas Meyer-Lindenberg shared last authorship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOC 26 kb)
Rights and permissions
About this article
Cite this article
Grimm, O., Gass, N., Weber-Fahr, W. et al. Acute ketamine challenge increases resting state prefrontal-hippocampal connectivity in both humans and rats. Psychopharmacology 232, 4231–4241 (2015). https://doi.org/10.1007/s00213-015-4022-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4022-y