Abstract
Rationale
Melatonin modifies physiological and behavioral responses to psychostimulants, with the MT1 and MT2 melatonin receptors specifically implicated in facilitating methamphetamine (METH)-induced sensitization in melatonin-proficient mice.
Objective
The objective of the study is to assess differences in locomotor sensitization after a single dose of methamphetamine in low-melatonin-expressing C57BL/6 wild-type and MT1 receptor knockout (MT1KO) mice, comparing with melatonin-expressing C3H/HeN mice.
Methods
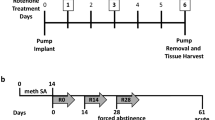
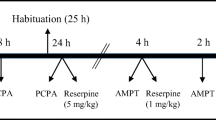
Mice received a vehicle or methamphetamine (1.2 mg/kg, i.p.) pretreatment (day 1) during the light (ZT5-9) or dark (ZT 19–21) periods in novel test arenas. Locomotor sensitization was assessed by methamphetamine challenge after an eight-day abstinence (day 9). TH protein expression was evaluated by immunofluorescence and Western blot analysis.
Results
Methamphetamine pretreatment induced statistically significant locomotor sensitization upon challenge after eight-day abstinence in C3H and C57 wild-type mice during the light period. The magnitude of sensitization in C57 mice was diminished in the dark period and completely abrogated in MT1KO mice. No differences were observed in tyrosine hydroxylase immunoreactivity in the mesolimbic dopamine system. Additional exposures to the test arenas after methamphetamine pretreatment (nights 2–6) enhanced sensitization.
Conclusions
Deletion of the MT1 melatonin receptor abolishes sensitization induced by a single METH pretreatment. The magnitude of sensitization is also altered by time of day and contextual cues. We conclude that the MT1 melatonin receptor is emerging as a novel target of therapeutic intervention for drug abuse disorders.





Similar content being viewed by others
References
Abarca C, Albrecht U, Spanagel R (2002) Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci USA 99:9026–9030
Addy NA, Daberkow DP, Ford JN, Garris PA, Wightman RM (2010) Sensitization of rapid dopamine signaling in the nucleus accumbens core and shell after repeated cocaine in rats. J Neurophysiol 104:922–931
Akhisaroglu M, Ahmed R, Kurtuncu M, Manev H, Uz T (2004) Diurnal rhythms in cocaine sensitization and in Period1 levels are common across rodent species. Pharmacol, Biochem Behav 79:37–42
Badiani A, Anagnostaras SG, Robinson TE (1995a) The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacology (Berl) 117:443–452
Badiani A, Browman KE, Robinson TE (1995b) Influence of novel versus home environments on sensitization to the psychomotor stimulant effects of cocaine and amphetamine. Brain Res 674:291–298
Bevins RA, Peterson JL (2004) Individual differences in rats' reactivity to novelty and the unconditioned and conditioned locomotor effects of methamphetamine. Pharmacol, Biochem Behav 79:65–74
Browning C, Beresford I, Fraser N, Giles H (2000) Pharmacological characterization of human recombinant melatonin mt(1) and MT(2) receptors. Br J Pharmacol 129:877–886
Chiu CT, Ma T, Ho IK (2005) Attenuation of methamphetamine-induced behavioral sensitization in mice by systemic administration of naltrexone. Brain Res Bull 67:100–109
Crusio WE, Goldowitz D, Holmes A, Wolfer D (2009) Standards for the publication of mouse mutant studies. Genes Brain Behav 8:1–4
Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J (2010) International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev 62:343–380
Dubocovich ML, Hudson RL, Sumaya IC, Masana MI, Manna E (2005) Effect of MT1 melatonin receptor deletion on melatonin-mediated phase shift of circadian rhythms in the C57BL/6 mouse. J Pineal Res 39:113–120
Dudai Y (2006) Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol 16:174–178
Ersahin C, Masana MI, Dubocovich ML (2002) Constitutively active melatonin MT(1) receptors in male rat caudal arteries. Eur J Pharmacol 439:171–172
Faith RE, Huerkamp MJ (2009) Environmental considerations for research animals. In: Hessler J, Lehner N (eds) Planning and designing research animal facilities. Elsevier, London, pp 59–83
Falcon E, McClung CA (2009) A role for the circadian genes in drug addiction. Neuropharmacology 56(Suppl 1):91–96
Futamura T, Akiyama S, Sugino H, Forbes A, McQuade RD, Kikuchi T (2010) Aripiprazole attenuates established behavioral sensitization induced by methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry 34:1115–1119
Gaytan O, Lewis C, Swann A, Dafny N (1999) Diurnal differences in amphetamine sensitization. Eur J Pharmacol 374:1–9
Gillette MU, Mitchell JW (2002) Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell Tissue Res 309:99–107
Hirabayashi M, Alam MR (1981) Enhancing effect of methamphetamine on ambulatory activity produced by repeated administration in mice. Pharmacol, Biochem Behav 15:925–932
Hirabayashi M, Okada S, Tadokoro S (1991) Comparison of sensitization to ambulation-increasing effects of cocaine and methamphetamine after repeated administration in mice. J Pharm Pharmacol 43:827–830
Hirata H, Asanuma M, Cadet JL (1998) Melatonin attenuates methamphetamine-induced toxic effects on dopamine and serotonin terminals in mouse brain. Synapse 30:150–155
Hutchinson AJ, Hudson RL, Dubocovich ML (2012) Genetic deletion of MT(1) and MT(2) melatonin receptors differentially abrogates the development and expression of methamphetamine-induced locomotor sensitization during the day and the night in C3H/HeN mice. J Pineal Res 53:399–409
Imbesi M, Arslan AD, Yildiz S, Sharma R, Gavin D, Tun N, Manev H, Uz T (2009) The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal ‘clock’ gene expression in striatal neurons in vitro. J Pineal Res 46:87–94
Itzhak Y, Martin JL, Black MD, Ali SF (1998) Effect of melatonin on methamphetamine- and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurotoxicity and methamphetamine-induced behavioral sensitization. Neuropharmacology 37:781–791
Kaewsuk S, Sae-ung K, Phansuwan-Pujito P, Govitrapong P (2009) Melatonin attenuates methamphetamine-induced reduction of tyrosine hydroxylase, synaptophysin and growth-associated protein-43 levels in the neonatal rat brain. Neurochem Int 55:397–405
Kasahara T, Abe K, Mekada K, Yoshiki A, Kato T (2010) Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci USA 107:6412–6417
Kongsuphol P, Mukda S, Nopparat C, Villarroel A, Govitrapong P (2009) Melatonin attenuates methamphetamine-induced deactivation of the mammalian target of rapamycin signaling to induce autophagy in SK-N-SH cells. J Pineal Res 46:199–206
Kopp C, Vogel E, Rettori MC, Delagrange P, Renard P, Lesieur D, Misslin R (1999) Regulation of emotional behaviour by day length in mice: implication of melatonin. Behav Pharmacol 10:747–752
Kozanian OO, Gutierrez A, Mohd-Yusof A, McDougall SA (2012) Ontogeny of methamphetamine-induced and cocaine-induced one-trial behavioral sensitization in preweanling and adolescent rats. Behav Pharmacol 23:367–379
Kurokawa K, Shibasaki M, Mizuno K, Ohkuma S (2011) Gabapentin blocks methamphetamine-induced sensitization and conditioned place preference via inhibition of alpha(2)/delta-1 subunits of the voltage-gated calcium channels. Neuroscience 176:328–335
Kurtuncu M, Arslan AD, Akhisaroglu M, Manev H, Uz T (2004) Involvement of the pineal gland in diurnal cocaine reward in mice. Eur J Pharmacol 489:203–205
Nikaido T, Akiyama M, Moriya T, Shibata S (2001) Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol Pharmacol 59:894–900
Orsini C, Buchini F, Piazza PV, Puglisi-Allegra S, Cabib S (2004) Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology (Berl) 172:264–270
Paxinos G, Franklin KB (2001) The mouse brain in stereotaxic coordinates. Academic, San Diego
Paz MC, Assis MA, Cabrera RJ, Cancela LM, Bregonzio C (2011) The AT(1) angiotensin II receptor blockade attenuates the development of amphetamine-induced behavioral sensitization in a two-injection protocol. Synapse 65:505–512
Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25:192–216
Rauhut AS, Bialecki V (2011) Development and persistence of methamphetamine-conditioned hyperactivity in Swiss-Webster mice. Behav Pharmacol 22:228–238
Reiter RJ (1991) Melatonin: the chemical expression of darkness. Mol Cell Endocrinol 79:C153–158
Robinson TE, Berridge KC (2008) Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci 363:3137–3146
Roka F, Brydon L, Waldhoer M, Strosberg AD, Freissmuth M, Jockers R, Nanoff C (1999) Tight association of the human Mel(1a)-melatonin receptor and G(i): precoupling and constitutive activity. Mol Pharmacol 56:1014–1024
Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC (1998) Natural melatonin ‘knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res 63:189–197
Shen X, Purser C, Tien LT, Chiu CT, Paul IA, Baker R, Loh HH, Ho IK, Ma T (2010) mu-Opioid receptor knockout mice are insensitive to methamphetamine-induced behavioral sensitization. J Neurosci Res 88:2294–2302
Soares JM Jr, Masana MI, Ersahin C, Dubocovich ML (2003) Functional melatonin receptors in rat ovaries at various stages of the estrous cycle. J Pharmacol Exp Ther 306:694–702
Stewart J, Badiani A (1993) Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol 4:289–312
Suwanjang W, Phansuwan-Pujito P, Govitrapong P, Chetsawang B (2010) The protective effect of melatonin on methamphetamine-induced calpain-dependent death pathway in human neuroblastoma SH-SY5Y cultured cells. J Pineal Res 48:94–101
Takino N, Sakurai E, Kuramasu A, Okamura N, Yanai K (2009) Roles of the histaminergic neurotransmission on methamphetamine-induced locomotor sensitization and reward: a study of receptors gene knockout mice. Int Rev Neurobiol 85:109–116
Torres-Farfan C, Seron-Ferre M, Dinet V, Korf HW (2006) Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J Pineal Res 40:64–70
Uz T, Akhisaroglu M, Ahmed R, Manev H (2003) The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology 28:2117–2123
Uz T, Javaid JI, Manev H (2002) Circadian differences in behavioral sensitization to cocaine: putative role of arylalkylamine N-acetyltransferase. Life Sci 70:3069–3075
Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Herve D, Girault JA (2010) Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology 35:401–415
Vezina P (2004) Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev 27:827–839
von Gall C, Lewy A, Schomerus C, Vivien-Roels B, Pevet P, Korf HW, Stehle JH (2000) Transcription factor dynamics and neuroendocrine signalling in the mouse pineal gland: a comparative analysis of melatonin-deficient C57BL mice and melatonin-proficient C3H mice. Eur J Neurosci 12:964–972
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492
Acknowledgments
The authors would like to thank Iwona Stepien, Kathleen McGowan, and Peter Crombe for their capable assistance with animal management and genotyping, and equipment maintenance.
Author contributions
All authors contributed to the conception, design, planning, data acquisition, and/or analysis of these studies. AJH drafted the manuscript which was revised and edited with substantial feedback from all authors. All authors approved the final version of the manuscript before submission.
Conflict of interest
This work was funded by R01DA021870 to MLD. The authors declare that over the last three years, MLD was a consultant for and received compensation from Takeda Pharmaceutical North America Inc.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
Locomotor Responses in C57 Mice Subjected to Six Daily VEH or METH Pretreatments. Experimental protocol is shown in Panel A. Ordinates on the line graphs represent distance traveled (mean ± SEM) in 2h tests as a function of Pretreatment Day (B) and in 5 min intervals as a function of time during the challenge test on Day 11 (C). *p < 0.05 when compared to VEH; ns denotes not significant. (JPEG 56 kb)
Supplemental Figure 2
TH Immunofluorescence in the Mesolimbic Dopamine System of C57 WT and MT1KO Mice. Representative images show CPu, NAc core, NAc shell and VTA for C57 WT (A–D) and MT1KO mice (E–H). TH protein expression was evaluated by fluorescence intensity (mean gray value, mean ± SEM) in the CPu, NAc core and NAc shell (I–K). Density of TH-positive cell bodies (mean ± SEM) was assessed in the VTA (L). Scale bar in Panel A corresponds to all CPu and NAc images. No significant (ns) differences were found between WT (n = 4) and MT1KO mice (n = 4). (JPEG 63 kb)
Supplemental Figure 3
TH Protein Levels in the Mesolimbic Dopamine System. Western blot analysis of brain homogenates from C57 WT (n = 8) and MT1KO mice (n = 8). Representative blot images are shown for CPu (A), NAc (B) and VTA (C) with corresponding semiquantitative densitometry analyses. Ordinates represent relative mean ± SEM of TH band intensity normalized to corresponding GAPDH values. No significant (ns) differences were observed between genotypes. (JPEG 42 kb)
Rights and permissions
About this article
Cite this article
Hutchinson, A.J., Ma, J., Liu, J. et al. Role of MT1 melatonin receptors in methamphetamine-induced locomotor sensitization in C57BL/6 mice. Psychopharmacology 231, 257–267 (2014). https://doi.org/10.1007/s00213-013-3228-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3228-0