Abstract
Rationale
Lack of benefit from antidepressant drug therapy is a major source of human suffering, affecting at least 25% of people with major depressive disorder. We want to know whether nonresponse to antidepressants can be linked to aberrant neuroreceptor binding.
Objective
This study aims to assess the antidepressant binding in brain regions of depressed nonresponders compared with healthy controls.
Materials and methods
Healthy volunteers and depressed subjects who had failed to benefit from at least 2 antidepressant treatments were recruited by newspaper advertisements. All subjects had received no antidepressant medication for at least 2 months before positron emission tomography (PET) that was carried out with [11C]mirtazapine. Kinetic parameters of [11C]mirtazapine were determined from PET data in selected brain regions by the simplified reference tissue model.
Results
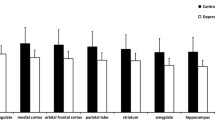
Binding potentials of [11C]mirtazapine in cerebral cortical regions were lower in depressed nonresponders than in healthy controls. Removal rates of [11C]mirtazapine were higher in diencephalic regions of depressed nonresponders than in healthy controls.
Conclusions
PET neuroimaging with [11C]mirtazapine showed aberrant neuroreceptor binding in brain regions of depressed subjects who had failed to benefit from treatment with antidepressant drugs.



Similar content being viewed by others
References
Anttila SA, Leinonen EV (2001) A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev 7:249–264
Bech P (1988) Rating scales for mood disorders: applicability, consistency and construct validity. Acta Psychiatr Neurol Scand 78:45–55
Bech P, Allerup P, Gram LF, Reisby N, Rosenberg R, Jacobsen O, Nagy A (1981) The Hamilton depression scale. Evaluation of objectivity using logistic models. Acta Psychiatr Scand 63:290–299
Beck AT, Ward C, Mendelson M (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:571
Berlim MT, Turecki G (2007) What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol 17:696–707
Bertelsen A, Brugha T, Tien AY (1998) Schedules for clinical assessment in neuropsychiatry. Version 2.1. Glossary. Differential definitions of SCAN items and commentary on the SCAN manual. World Health Organization Division of Mental Health, Geneva
Berton O, Nestler EJ (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–151
Blier P (2003) The pharmacology of putative early-onset antidepressant strategies. Eur Neuropsychopharmacol 13:57–66
Collignon A, Maes F, Delaere D, Vandermeulen D, Suetens P, Marchal G (1995) Automated multimodality image registration using information theory. In: Bizais Y, Barillot C, DiPaola R (eds) Information processing in medical imaging. Kluwer, Dordrecht, pp 263–274
Collins DL, Neelin P, Peter TM, Evans AC (1994) Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18:192–205
de Boer T (1996) The pharmacologic profile of mirtazapine. J Clin Psychiatry 57(Suppl 4):19–25
de Boer TH, Maura G, Raiteri M, de Vos CJ, Wieringa J, Pinder RM (1988) Neurochemical and autonomic pharmacological profiles of the 6-aza-analogue of mianserin, Org 3770 and its enantiomers. Neuropharmacology 27:399–408
Drevets WC, Savitz J, Trimble M (2008) The subgenual anterior cingulate cortex in mood disorders. CNS Spectr 13:663–681
Fava M (2003) Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 53:649–659
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 12:62
Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS (2005) Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder. Ann Intern Med 143:415–426
Hercher C, Turecki G, Mechawar N (2009) Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J Psychiatr Res (in press)
Kabani N, MacDonald D, Holmes C, Evans AC (1998) 3D Atlas of the human brain. In: Fourth International Conference on Functional Mapping of the Human Brain, Montreal, Quebec, Canada
Kent JM (2003) SNaRIs, NaSSAs, and NaRIs: new agents for the treatment of depression. Lancet 355:911–918
Krishnan V, Nestler EJ (2008) The molecular neurobiology of depression. Nature 455:894–902
Lammertsma AA, Hume SP (1996) Simplified reference tissue model for PET receptor studies. NeuroImage 4:153–158
Marthi K, Bender D, Gjedde A, Smith D (2002) [11C]Mirtazapine for PET neuroimaging: radiosynthesis and initial evaluation in the living porcine brain. Eur Neuropsychopharmacol 12:427–432
Marthi K, Jakobsen S, Bender D, Hansen SB, Smith SB, Hermansen F, Rosenberg R, Smith DF (2004) [N-methyl-11C]Mirtazapine for positron emission tomography neuroimaging of antidepressant actions in humans. Psychopharmacology (Berl) 174:260–265
Meyer JH (2007) Imaging the serotonin transporter during major depressive disorder and antidepressant treatment. J Psychiatry Neurosci 32:86–102
Meyer JH, Wilson AA, Ginovart N, Goulding V, Hussey D, Hood K, Houle S (2001) Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11) C]DASB PET imaging study. Am J Psychiatry 158:1843–1849
Millan MJ (2006) Multi-target strategies for the improved treatment of depressive states: conceptual foundations and neuronal substrates, drug discovery and therapeutic application. Pharmacol Ther 110:135–370
Millan MJ, Gobert A, Rivet JM, Adhumeau-Auclair A, Cussac D, Newman-Tancredi A, Dekeyne A, Nicolas JP, Lejeune F (2000) Mirtazapine enhances frontocortical dopaminergic and corticolimbic adrenergic, but not serotonergic, transmission by blockade of alpha2-adrenergic and serotonin2C receptors: a comparison with citalopram. Eur J NeuroSci 12:1079–1095
Montgomery SA, Reimitz PE, Zivkov M (1998) Mirtazapine versus amitriptyline in the long-term treatment of depression: a double-blind placebo-controlled study. Int Clin Psychopharmacol 13:63–73
Montgomery SA, Baldwin DS, Blier P, Fineberg NA, Kasper S, Lader M, Lam RW, Lepine JP, Moller HJ, Nutt DJ, Rouillon F, Schatzberg AF, Thase ME (2007) Which antidepressants have demonstrated superior efficacy? A review of the evidence. Int Clin Psychopharmacol 22:323–329
Oquendo MA, Mann JJ (2000) The biology of impulsivity and suicidality. Psychiatr Clin North Am 23:11–25
Papakostas GI, Thase ME, Fava M, Craig NJ, Shelton RC (2007) Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? A meta-analysis of studies of newer agents. Biol Psychiatry 62:1217–1227
Parker G (2000) Classifying depression: should paradigms lost be regained? Am J Psychiatry 157:1195–1203
Petersen T, Papakostas GI, Posternak MA, Kant A, Guyker WM, Iosifescu DV, Yeung AS, Nierenberg AA, Fava M (2005) Empirical testing of two models for staging antidepressant treatment resistance. J Clin Psychopharmacol 25:336–341
Quitkin FM, Taylor BP, Kremer C (2001) Does mirtazapine have a more rapid onset than SSRIs? J Clin Psychiatry 62:358–361
Ressler KJ, Mayberg HS (2007) Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10:1116–1124
Rush AJ, Thase ME, Dube S (2003) Research issues in the study of difficult-to-treat depression. Biol Psychiatry 53:743–753
Rush AJ, Wisniewski SR, Warden D, Luther JF, Davis LL, Fava M, Nierenberg AA, Trivedi MH (2008) Selecting among second-step antidepressant medication monotherapies: predictive value of clinical, demographic, or first-step treatment features. Arch Gen Psychiatry 65:870–880
Russell JM, Hawkins K, Ozminkowski RJ, Orsini L, Crown WH, Kennedy S, Finkelstein S, Berndt E, Rush AJ (2004) The cost consequences of treatment-resistant depression. J Clin Psychiatry 65:341–347
Sadzot B, Lemaire C, Maquet P, Salmon E, Plenevaux A, Degueldre C, Hermanne JP, Guillaume M, Cantineau R, Comar D et al (1995) Serotonin 5HT2 receptor imaging in the human brain using positron emission tomography and a new radioligand, [18F]altanserin: results in young normal controls. J Cereb Blood Flow Metab 15:787–797
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97
Smith DF, Jakobsen S (2009) Molecular tools for assessing human depression by positron emission tomography. Eur Neuropsychopharmacol (in press)
Smith DF, Dyve S, Minuzzi L, Jakobsen S, Munk OL, Marthi K, Cumming P (2006) Inhibition of [11C]mirtazapine binding by alpha2-adrenoceptor antagonists studied by positron emission tomography in living porcine brain. Synapse 59:463–471
Smith DF, Stork BS, Wegener G, Jakobsen S, Bender D, Audrain H, Jensen SB, Hansen SB, Rodell A, Rosenberg R (2007) Receptor occupancy of mirtazapine determined by PET in healthy volunteers. Psychopharmacology (Berl) 195:131–138
Stockmeier CA (2003) Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res 37:357–373
Szegedi A, Schwertfeger N (2005) Mirtazapine: a review of its clinical efficacy and tolerability. Expert Opin Pharmacother 6:631–641
Tabachnick BG, Fidell LS (1989) Using multivariate statistics. HarperCollins, New York
Wheatley DP, van Moffaert M, Timmerman L, Kremer CM (1998) Mirtazapine: efficacy and tolerability in comparison with fluoxetine in patients with moderate to severe major depressive disorder. Mirtazapine–Fluoxetine Study Group. J Clin Psychiatry 59:306–312
Yasuno F, Hasnine AH, Suhara T, Ichimiya T, Sudo Y, Inoue M, Takano A, Ou T, Ando T, Toyama H (2002) Template-based method for multiple volumes of interest of human brain PET images. NeuroImage 16:577–586
Zhou Y, Endres CJ, Brasic JR, Huang SC, Wong DF (2003) Linear regression with spatial constraint to generate parametric images of ligand-receptor dynamic PET studies with a simplified reference tissue model. NeuroImage 18:975–989
Acknowledgments
We thank the Danish Medical Research Council, A.P. Møller Foundation for the Advancement of Medical Science, and Max and Inger Woerzner Foundation for financial support and everyone at the PET Center of Aarhus University Hospital for providing a positive research environment.
Authors’ contributions
All authors participated in producing the final written report. D.F.S. designed and performed all experiments, analyzed the data, and wrote the first draft of the manuscript. B.S.S., G.W., and M.A. examined subjects, injected radioligand, and performed clinical duties related to safety and health of volunteers. S.J., D.B., H.A., and K.H.V. produced the radioligand in accordance with quality control requirements. S.B.H. was responsible for technical equipment and data registration. P.V. and R.R. assisted in designing experiments and were responsible for clinical issues as required by the Danish Medicines Agency and the local ethical committee.
Competing interests
The authors declare that they have no competing financial interests related to this report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, D.F., Stork, B.S., Wegener, G. et al. [11C]Mirtazapine binding in depressed antidepressant nonresponders studied by PET neuroimaging. Psychopharmacology 206, 133–140 (2009). https://doi.org/10.1007/s00213-009-1587-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1587-3