Abstract
Rationale
Chronic treatment with neuroleptics causes, as a side effect, tardive dyskinesia in humans; however, the mechanisms involved in its pathophysiology remain unclear.
Objectives
The purpose of this study was to examine the effects of diphenyl diselenide, an organoselenium compound with antioxidant properties, in an animal model of vacuous chewing movements (VCMs) induced by long-term treatment with fluphenazine.
Results
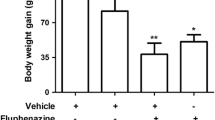
Adult male rats were treated during 24 weeks with fluphenazine (25 mg/kg, intramuscularly [i.m.], once every 21 days) and diphenyl diselenide (1 mg/kg, subcutaneously, three times a week). VCMs and body weight gain were quantified every 3 weeks. The fluphenazine treatment produced VCMs in the majority of the treated rats (87% after 24 weeks). Concomitant treatment with diphenyl diselenide decreased the prevalence of VCMs to 50%. Additionally, we separated the rats that developed or did not develop VCMs. We did not find any statistical differences among the groups when oxidative stress parameters were evaluated. Chronic fluphenazine treatment significantly decreased [3H]-dopamine uptake. Concomitant treatment with diphenyl diselenide was not able to prevent this decrease in those rats that developed VCMs.
Conclusions
Our data suggest that the reduction in dopamine transport can be a possible mechanism related to the maintenance of VCMs in rats. Moreover, diphenyl diselenide seems to be a promising pharmacological agent in the reduction in the prevalence of VCMs in rats.



Similar content being viewed by others
References
Abílio VC, Silva RH, Carvalho RC, Grassl C, Calzavara MB, Registro S, D’Almeida V, Ribeiro RA, Frussa-Filho R (2004) Important role of striatal catalase in aging- and reserpine-induced oral dyskinesia. Neuropharmacology 47:263–272
Adler LA, Rotrosen J, Edson R, Lavori P, Lohr J, Hitzemann R, Raisch D, Caliguri M, Tracy K (1999) Vitamin E treatment for tardive dyskinesia. Arch Gen Psychiatry 56:836–841
Andreassen OA, Jorgensen HA (2000) Neurotoxicity associated with neuroleptic-induced oral dyskinesias in rats. Implications for tardive dyskinesia? Prog Neurobiol 61:525–541
Andreassen OA, Ferrante RJ, Aamo TO, Beal MF, Jorgensen HA (2003) Oral dyskinesias and histopathological alterations in substantia nigra after long-term haloperidol treatment of old rats. Neuroscience 122:717–725
Avent KM, Usuki E, Eyles DW, Keeve R, Van der Schyf CJ, Castagnoli N Jr, Pond SM (1996) Haloperidol and its tetrahydropyridine derivative (HPTP) are metabolized to potentially neurotoxic pyridinium species in the baboon. Life Sci 59:1473–1482
Borges LP, Borges VC, Moro AV, Nogueira CW, Rocha JB, Zeni G (2005) Protective effect of diphenyl diselenide on acute liver damage induced by 2-nitropropane in rats. Toxicology 210:1–8
Boyson SJ, McGonigle P, Luthin GR, Wolfe BB, Molinoff PB (1988) Effects of chronic administration of neuroleptic and anticholinergic agents on densities of D2 dopamine and muscarinic cholinergic receptors in rat striatum. J Pharmacol Exp Ther 244:987–993
Brandão R, Lara FS, Pagliosa LB, Soares FA, Rocha JB, Nogueira CW, Farina M (2005) Hemolytic effects of sodium selenite and mercuric chloride in human blood. Drug Chem Toxicol 28:397–407
Brenneisen P, Steinbrenner H, Sies H (2005) Selenium, oxidative stress, and health aspects. Mol Aspects Med 26:256–267
Brown K, Reid A, White T, Henderson T, Hukin S, Johnstone C, Glen A (1998) Vitamin E, lipids, and lipid peroxidation products in tardive dyskinesia. Biol Psychiatry 43:863–867
Burger ME, Fachinetto R, Calegari L, Paixão MW, Braga AL, Rocha JBT (2004) Effects on age on orofacial dyskinesia reserpine-induced and possible protection of diphenyl-diselenide. Brain Res Bull 64:339–345
Burger ME, Fachinetto R, Zeni G, Rocha JBT (2005a) Ebselen attenuates haloperidol-induced orofacial dyskinesia and oxidative stress in rat brain. Pharmacol Biochem Behav 81:608–615
Burger ME, Fachinetto R, Alves A, Calegari L, Rocha JBT (2005b) Acute reserpine and subchronic haloperidol treatments change synaptosomal brain glutamate uptake and elicit orofacial dyskinesia in rats. Brain Res 1031:202–210
Burger MB, Fachinetto R, Wagner C, Perottoni J, Pereira RP, Zeni G, Rocha JBT (2006) Effects of diphenyl-diselenide on orofacial dyskinesia model in rats. Brain Res Bull 70:165–170
Burt D, Creese I, Snyder SH (1977) Antischizophrenic drugs: chronic treatment elevates dopamine receptor binding in brain. Science 196:326–327
Cadet JL, Lohr JB, Jeste DV (1986) Free radical and tardive dyskinesia. Trends Neurosci 9:107–108
Cadet JL, Lohr JB, Jeste DV (1987) Tardive dyskinesia and Schizophrenic burnout: the possible involvement of cytotoxic free radicals. In: Henn FA, DeLisi LE (eds) Handbook of schizophrenia: the neurochemistry and pharmacology of schizophrenia. Elsevier, Amsterdam, pp 425–438
Casey DE (1985) Tardive dyskinesia: reversible and irreversible. Psychopharmacology 2:88–97
Chang LW (1983) Protective effects of selenium against methylmercury neurotoxicity: a morphological and biochemical study. Exp Pathol 23:143–156
Commandeur JNM, Rooseboom M, Vermeulen NPE (2001) Chemistry and biological activity of novel selenium-containing compounds. Biological reactive intermediates VI. Adv Exp Med Biol 500:105
Crane GE (1973) Persistent dyskinesia. Br J Psychiatry 122:395–405
Dabiri LM, Pasta D, Darby JK, Mosbacher D (1994) Effectiveness of vitamin E for treatment of long-term tardive dyskinesia. Am J Psychiatry 151:925–926
Ebadi M, Srinivasan SK (1995) Pathogenesis, prevention and treatment of neuroleptic-induced movement disorders. Pharmacol Rev 47:575–604
Egan MF, Hyde TM, Albers GW, Elkashef A, Alexander RC, Reeve A, Blum A, Saenz RE, Wyatt RJ (1992) Treatment of tardive dyskinesia with vitamin E. Am J Psychiatry 149:773–777
Egan MF, Hurd Y, Hyde TM, Weinberger DR, Wyatt RJ, Kleinman JE (1994) Alterations in mRNA levels of D2 receptors and neuropeptides in striatonigral and striatopallidal neurons of rats with neuroleptic-induced dyskinesias. Synapse 18:178–189
Egan MF, Hurd Y, Ferguson J, Bachus SE, Hamid EH, Hyde TM (1996) Pharmacological and neurochemical differences between acute and tardive vacuous chewing movements induced by haloperidol. Psychopharmacology 127:337–345
Fachinetto R, Burger MB, Wagner C, Wondracek DC, Brito VB, Nogueira CW, Ferreira J, Rocha JBT (2005) High fat diet increases the incidence of orofacial dyskinesia and oxidative stress in specific brain regions of rats. Pharmacol Biochem Behav 81:585–592
Faria RR, Abilio VC, Grassl C, Chinen CC, Negrao LT, de Castro JP, Fukushiro DF, Rodrigues MS, Gomes PH, Registro S, de Carvalho RC, D’Almeida V, Silva RH, Ribeiro RA, Frussa-Filho R (2005) Beneficial effects of vitamin C and vitamin E on reserpine-induced oral dyskinesia in rats: critical role of striatal catalase activity. Neuropharmacology 48:993–1001
Gerlach J, Casey D (1988) Tardive dyskinesia. Acta Psychiatr Scand 77:369–378
Gunne L, Häggström J, Sjöquist B (1984) Association with persistent neuroleptic-induced dyskinesia of regional changes in brain GABA synthesis. Nature 309:347–349
Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Shimizu E, Iyo M (2004) Protective effects of N-acetyl-L-cysteine on the reduction of dopamine transporters in the striatum of monkeys treated with methamphetamine. Neuropsychopharmacology 29:2018–2023
Holz RW, Coyle JT (1974) The effects of various salts, temperature and the alkaloids veratridine and batrachotoxin on the uptake of 3H-dopamine into synaptosomes from rat striatum. Mol Pharmacol 10:746–758
Horn AS (1990) Dopamine uptake: a review of progress in the last decade. Prog Neurobiol 34:387–400
Huang CL, Huang NK, Shyue SK, Chern Y (2003) Hydrogen peroxide induces loss of dopamine transporter activity: a calcium-dependent oxidative mechanism. J Neurochem 86:1247–1259
Iversen LL (1971) Role of transmitter uptake mechanisms in synaptic neurotransmission. Br J Pharmacol 41:571–591
Jackson-Lewis V, Przedborski S, Kostic V, Suber F, Fahn S, Cadet JL (1991) Partial attenuation of chronic fluphenazine-induced changes in regional monoamine metabolism by D-alpha-tocopherol in rat brain. Brain Res Bull 26:251–258
Jeding I, Evans PJ, Akanmu D, Dexter D, Spencer JD, Aruoma OI, Jenner P, Halliwell B (1995) Characterization of the potential antioxidant and pro-oxidant actions of some neuroleptic drugs. Biochem Pharmacol 49:359–365
Jenner P, Marsden CD (1983) Neuroleptics and tardive dyskinesia. In: Coyle JT, Enna SJ (eds) Neuroleptics: neurochemical, behavioral and, clinical perspectives. Raven Press, New York, pp 223–253
Jeste DV, Potkin SG, Sinhá S, Feder S, Wyatt RJ (1979) Tardive dyskinesia—reversible and persistent. Arch Gen Psychiatry 36:585–590
Jeste DV, Lohr JB, Manley M (1992) Study of neuropathologic changes in the striatum following 4, 8 and 12 months of treatment with fluphenazine in rats. Psychopharmacology 106:154–160
Kane JM (1995) Tardive dyskinesia: epidemiology and clinical presentation. In: Bloom FE, Kupfer DJ (eds) Psychopharmacology: the fourth generation of progress, vol. 39. Raven Press, New York 1485–1495
Kane JM, Smith JM (1982) Tardive dyskinesia: prevalence and risk factors, 1959 to 1979. Arch Gen Psychiatry 39:473–481
Klotz LO, Kroncke KD, Buchczyk DP, Sies H (2003) Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J Nutr 133:1448
Lee S, Oh D, Jung S, Kim Y, Cho H, Koh J, Lee Y (1997) Neuroleptic drugs alter the dopamine transporter-mediated uptake and release of dopamine: a possible mechanism for drug-induced tardive dyskinesia. Pharmacol Res 35:446–450
Lohr JB, Cadet JL, Lohr MA, Jeste DV, Wyatt RJ (1987) Alpha-tocopherol in tardive dyskinesia. Lancet 1:913–934
Lohr JB, Kuczenski R, Bracha HS, Moir M, Jeste DV (1990) Increased indices of free radical activity in the cerebrospinal fluid of patients with tardive dyskinesia. Biol Psychiatry 28:535–539
Lohr JB, Caligiuri MP, Manley MS, Browning JA (2000) Neuroleptic-induced striatal damage in rats: a study of antioxidant treatment using accelerometric and immunocytochemical methods. Psychopharmacology 148:171–179
Lohr JB, Kuczenski R, Niculescu AB (2003) Oxidative mechanisms and tardive dyskinesia. CNS Drugs 17:47–62
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265
Mehler-Wex C, Riederer P, Gerlach M (2006) Dopaminergic dysbalance in distinct basal ganglia neurocircuits: implications for the pathophysiology of Parkinson’s disease, schizophrenia and attention deficit hyperactivity disorder. Neurotox Res 10:167–179
Miczek KA, Fish EW, De Bold JF, De Almeida RM (2002) Social and neural determinants of aggressive behavior: pharmacotherapeutic targets at serotonin, dopamine and gamma-aminobutyric acid systems. Psychopharmacology 163:434–458
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Naidu PS, Singh A, Kaur P, Kulkarni SK (2003) Quercetin, a bioflavonoid, attenuates haloperidol-induced orofacial dyskinesia. Neuropharmacology 44:1100–1106
Nogueira CW, Rotta LN, Perry ML, Souza DO, Rocha JBT (2001) Diphenyl diselenide and diphenyl ditelluride affect the rat glutamatergic system in vitro and in vivo. Brain Res 906:157–163
Nogueira CW, Meotti FC, Curte E, Pilissão C, Zeni G, Rocha JBT (2003) Investigations into the potencial neurotoxicity induced by diselenides in mice and rats. Toxicology 183:29–37
Nogueira CW, Zeni G, Rocha JBT (2004) Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev 104:6255–6286
Ohkawa H, Ohishi H, Yagi K (1979) Assay for lipid peroxide in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pall HS, Williams AC, Blake DR, Lunee J (1987) Evidence of enhanced lipid peroxidation in the cerebrospinal fluid of patients taking phenothiazines. Lancet 2:596–599
Palmier C (1986) Selenium reagents and intermediates. In: Organic synthesis. Pergamon, Oxford
Pérez-Severiano P, Rodríguez-Pérez M, Pedraza-Chaverrí J, Maldonado PD, Medina-Campos ON, Ortíz-Plata A, Sánchez-García A, Villeda-Hernández J, Galván-Arzate S, Aguilera P, Santamaría A (2004) S-Allylcysteine, a garlic-derived antioxidant, ameliorates quinolinic acid-induced neurotoxicity and oxidative damage in rats. Neurochem Int 45:1175–1183
Post A, Rucker M, Ohl F, Uhr M, Holsboer F, Almeida OFX, Michaelidis TM (2002) Mechanisms underlying the protective potential of alpha-tocopherol (vitamin E) against haloperidol-associated neurotoxicity. Neuropsychopharmacology 26:397–407
Sachdev P, Saharov T, Cathcart S (1999) The preventative role of antioxidants (selegiline and vitamin E) in a rat model of tardive dyskinesia. Biol Psychiatry 46:1672–1681
Sadan O, Bahat-Stromza M, Gilgun-Sherki Y, Atlas D, Melamed E, Offen D (2005) A novel brain-targeted antioxidant (AD4) attenuates haloperidol-induced abnormal movement in rats: implications for tardive dyskinesia. Clin Neuropharmacol 28:285–288
Santos FW, Zeni G, Rocha JB, Weis SN, Fachinetto JM, Favero AM, Nogueira CW (2005) Diphenyl diselenide reverses cadmium-induced oxidative damage on mice tissues. Chem Biol Interact 151:159–165
See RE (1993) Assessment of striatal extracellular dopamine and dopamine metabolites by microdialysis in haloperidol-treated rats exhibiting oral dyskinesia. Neuropsychopharmacology 9:101–109
Shirakawa O, Tamminga CA (1994) Basal ganglia GABAA and dopamine D1 binding site correlates of haloperidol-induced oral dyskinesias in rat. Exp Neurol 127:62–69
Shivakumar BR, Ravindratnath V (1993) Oxidative stress and thiol modification induced by chronic administration of haloperidol. J Pharmacol Exp Ther 265:1137–1141
Steinbrenner H, Alili L, Bilgic E, Sies H, Brenneisen P (2006) Involvement of selenoprotein P in protection of human astrocytes from oxidative damage. Free Radic Biol Med 40:1513–1523
Tsai G, Goff DC, Chang RW, Flood J, Baer L, Coyle JT (1998) Markers of glutamatergic neurotransmission and oxidative stress associated with tardive dyskinesia. Am J Psychiatry 155:1207–1213
van Erp AM, Miczek KA (2007) Increased accumbal dopamine during daily alcohol consumption and subsequent aggressive behavior in rats. Psychopharmacology 191:679–688
Van Kampen JM, Stoessl AJ (2000) Dopamine D1A receptor function in a rodent model of tardive dyskinesia. Neuroscience 101:629–635
Westfall TC, Besson M, Giorguieff M, Glowinski J (1976) The role of presynaptic receptors in the release and synthesis of 3H-dopamine by slices of rat striatum. Arch Pharmacol 292:279–287
Woerner MG, Kane JM, Lieberman JA, Alvir J, Bergmann KJ, Borenstein M, Schooler NR, Mukherjee S, Rotrosen J, Rubinstein M et al (1991) The prevalence of tardive dyskinesia. J Clin Psychopharmacol 11:34–42
Wright AM, Bempong J, Kirby ML, Barlow RL, Bloomquist JR (1998) Effects of haloperidol metabolites on neurotransmitter uptake and release: possible role in neurotoxicity and tardive dyskinesia. Brain Res 788:215–222
Zafar KS, Siddiqui A, Sayeed I, Ahmad M, Salim S, Islam F (2003) Dose-dependent protective effect of selenium in rat model of Parkinson’s disease: neurobehavioral and neurochemical evidences. J Neurochem 84:438–446
Acknowledgments
The financial support by FAPERGS, CAPES, CNPq, and Cristália-SP is gratefully acknowledged. R. Fachinetto, J. G. Villarinho, R. P. Pereira, C. Wagner, and J.B.T. Rocha (no. 120066/2005-0) were recipients of the CNPq fellowship. R. Fachinetto was a recipient of the CAPES fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fachinetto, R., Villarinho, J.G., Wagner, C. et al. Diphenyl diselenide decreases the prevalence of vacuous chewing movements induced by fluphenazine in rats. Psychopharmacology 194, 423–432 (2007). https://doi.org/10.1007/s00213-007-0831-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0831-y