Abstract
Rationale
Conditioned place aversion (CPA) is known to be a sensitive measure of the aversive motivational state produced by opioid withdrawal in rats made chronically dependent on opioids.
Objective
The purpose of the present study was to examine the sensitivity of the CPA model in detecting a possible aversive state associated with naloxone-precipitated withdrawal from acute treatment with morphine.
Methods
Doses of morphine and naloxone, as well as number of conditioning trials, were systematically varied to determine the minimum conditions that would result in a detectable CPA in male Wistar rats. Naloxone (0.003–16.7 mg/kg) was administered 4 h after an injection of vehicle or morphine (1.0, 3.3, or 5.6 mg/kg) and immediately prior to confinement to one compartment of the conditioning apparatus; rats received either one or two such naloxone-conditioning trials (separate by 48 h).
Results
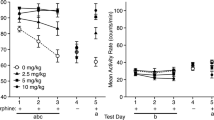
Morphine (5.6 mg/kg) followed 4 h later by vehicle produced no significant preference or aversion. In morphine-naive rats, 10 mg/kg naloxone was required to produce a significant CPA with two cycles of conditioning. When increasing doses of morphine were administered (1.0, 3.3, 5.6 mg/kg), significant increases in naloxone potency to elicit a CPA were observed (16-, 211-, and 1018-fold potency shifts, respectively). Naloxone potency after two pretreatments with 5.6 mg/kg morphine was comparable to its potency to elicit a CPA after chronic exposure to morphine. Although naloxone was still effective in producing a CPA after a single conditioning cycle (and hence a single morphine exposure), its effects were dramatically reduced relative to those seen with two conditioning cycles.
Conclusions
CPA is a reliable and sensitive index of the aversive motivational state accompanying withdrawal from acute opioid dependence.


Similar content being viewed by others
References
Adams JU, Holtzman SG (1990) Pharmacologic characterization of the sensitization to the rate-decreasing effects of naltrexone induced by acute opioid pretreatment in rats. J Pharmacol Exp Ther 253:483–489
Azorlosa JL, Stitzer ML, Greenwald MK (1994) Opioid physical dependence development: effect of single versus repeated morphine pretreatments and of subjects' opioid exposure history. Psychopharmacology 114:71–80
Bickel WK, Stitzer ML, Liebson IA, Bigelow GE (1988) Acute physical dependence in man: effects of naloxone after brief morphine exposure. J Pharmacol Exp Ther 244:126–132
Cheney DL, Goldstein A (1971) Tolerance to opioid narcotics: time course and reversibility of physical dependence in mice. Nature 232:477–478
Delfs JM, Zhu Y, Druhan JP, Aston-Jones G (2000) Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature 403:430–434
Easterling KW, Holtzman SG (1997) Intracranial self-stimulation in rats: sensitization to an opioid antagonist following acute or chronic treatment with mu opioid agonists. J Pharmacol Exp Ther 281:188–199
Easterling KW, Plovnick RM, Holtzman SG (2000) Acute opioid but not benzodiazepine dependence in rats responding for intracranial self-stimulation. Psychopharmacology 148:263–271
Gellert VF, Sparber SB (1977) A comparison of the effects of naloxone upon body weight loss and suppression of fixed-ratio operant behavior in morphine- dependent rats. J Pharmacol Exp Ther 201:44–54
Gracy KN, Dankiewicz LA, Koob GF (2001) Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of conditioned place aversion. Neuropsychopharmacology 24:152–160
Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA (1989a) Acute opioid physical dependence in humans: effect of varying the morphine-naloxone interval. I. J Pharmacol Exp Ther 250:485–491
Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA (1989b) Acute opioid physical dependence in postaddict humans: naloxone dose effects after brief morphine exposure. J Pharmacol Exp Ther 248:127–134
Heishman SJ, Stitzer ML, Bigelow GE, Liebson IA (1990) Acute opioid physical dependence in humans: effect of naloxone at 6 and 24 hours postmorphine. Pharmacol Biochem Behav 36:393–399
Jacob JJ, Michaud GM (1974) Acute physical dependence in the waking dog after a single dose of morphine. Psychol Med 4:270–273
Jacob JJC, Barthelemy CD, Tremblay EC, Colombel ML (1974) Potential usefulness of single-dose acute physical dependence on and tolerance to morphine for the evaluation of narcotic antagonists. Adv Biochem Psychopharmacol 8:299–318
Jones RT (1980) Dependence in non-addict humans after a single dose of morphine. In: Way EL (ed) Endogenous and exogenous opiate agonists and antagonists. Pergamon Press, New York, pp 557–560
Kirby KC, Stitzer ML (1993) Opioid physical dependence development in human: effect of time between agonist pretreatments. Psychopharmacology 112:511–517
Kirby KC, Stitzer ML, Heishman SJ (1990) Acute opioid physical dependence in humans: effect varying the morphine-naloxone interval II. J Pharmacol Exp Ther 255:730–737
Kosersky DS, Harris RA, Harris LS (1974) Naloxone-precipitated jumping activity in mice following the acute administration of morphine. Eur J Pharmacol 26:122–124
Liu J, McElfresh A, Reis S, Fuqua L, Schulteis G (2002) Neuroadaptive processes in limbic and basal forebrain reward circuitry contribute to acute opioid dependence. 2002 Abstract Viewer/Itinerary Planner, Society for Neuroscience, Washington D.C., CD-ROM, program no. 310.10
Martin WR, Eades CG (1964) A comparison between acute and chronic physical dependence in the chronic spinal dog. J Pharmacol Exp Ther 146:385–394
Meyer DR, Sparber SB (1977) Evidence of possible opiate dependence during the behavioral depressant action of a single dose of morphine. Life Sci 21:1087–1094
Mucha RF (1987) Is the motivational effect of opiate withdrawal reflected by common somatic indices of precipitated withdrawal? A place conditioning study in the rat. Brain Res 418:214–220
Mucha RF, Herz A (1985) Motivational properties of kappa and mu opioid receptor agonists. Psychopharmacology 86:274–280
Mucha RF, Iversen SD (1984) Reinforcing properties of morphine and naloxone revealed by conditioned place preferences: a procedural examination. Psychopharmacology 82:241–247
Mucha RF, Millan MJ, Herz A (1985) Aversive properties of naloxone in non-dependent (naive) rats may involve blockade of central beta-endorphin. Psychopharmacology 86:281–285
Mucha RF, Gritti MD, Kim C (1986) Aversive properties of opiate withdrawal studied in rats. In: Holaday JW, Law P, Herz A (eds) Progress in opioid research. NIDA Res Monogr, pp 567–570
Nikpur J, Morse AC, Britton KT, Schulteis G (2000) Acute opioid dependence is characterized by brain reward deficits. Soc Neurosci Abstr 26:1576
Parker LA, Joshi A (1998) Naloxone-precipitated morphine withdrawal induced place aversions: effect of naloxone at 24 hours postmorphine. Pharmacol Biochem Behav 61:331–333
Parker LA, Cyr JA, Santi AN, Burton PD (2002) The aversive properties of acute morphine dependence persist 48 h after a single exposure to morphine. Evaluation by taste and place conditioning. Pharmacol Biochem Behav 72:87–92
Ramabadran K (1983) Naloxone-precipitated abstinence in mice, rats and gerbils acutely dependent on morphine. Life Sci 33:385–388
Ritzmann RF (1981) Opiate dependence following acute injections of morphine and naloxone: the assessment of various withdrawal signs. Pharmacol Biochem Behav 14:575–577
Santi AN, Parker LA (2001) The dopamine antagonist, alpha-flupenthixol, interferes with naloxone-induced place aversion learning, but not with acute opiate dependence in rats. Pharmacol Biochem Behav 70:193–197
Schnur P, Espinoza M, Flores R, Ortiz S, Vallejos S, Wainwright M (1992) Blocking naloxone-precipitated withdrawal in rats and hamsters. Pharmacol Biochem Behav 43:1093–1098
Schulteis G, Markou A, Gold LH, Stinus L, Koob GF (1994) Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther 271:1391–1398
Schulteis G, Heyser CJ, Koob GF (1997) Opiate withdrawal signs precipitated by naloxone following a single exposure to morphine: potentiation with a second morphine exposure. Psychopharmacology 129:56–65
Schulteis G, Heyser CJ, Koob GF (1999) Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol 10:235–242
Smits SE (1975) Quantitation of physical dependence in mice by naloxone-precipitated jumping after a single dose of morphine. Res Commun Chem Pathol Pharmacol 10:651–661
Sofuoglu M, Sato J, Takemori AE (1990) Maintenance of morphine dependence by naloxone in acutely dependent mice. J Pharmacol Exp Ther 254:841–846
Stinus L, Le MM, Koob GF (1990) The nucleus accumbens and amygdala as possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience 37:767–773
Tallarida RJ, Murray RB (1986) Manual of pharmacologic calculations with computer programs. Springer, Berlin, Heidelberg, New York
Walker JR, Ahmed SH, Gracy KN, Koob GF (2000) Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. Brain Res 854:85–92
White-Gbadebo D, Holtzman SG (1994) Acute sensitization to opioid antagonists. Pharmacol Biochem Behav 47:559–566
Wiley JN, Downs DA (1979) Naloxone-precipitated jumping in mice pretreated with acute injections of opioids. Life Sci 25:797–802
Young AM (1986) Effects of acute morphine pretreatment on the rate-decreasing and antagonist activity of naloxone. Psychopharmacology 88:201–208
Acknowledgements
This research was supported by NIH grant DA-10475 (to G.S). Salary support for M.R.A. was provided by T32-DA-07277.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azar, M.R., Jones, B.C. & Schulteis, G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology 170, 42–50 (2003). https://doi.org/10.1007/s00213-003-1514-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1514-y