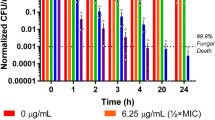
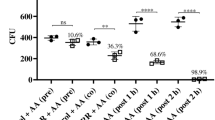
Abstract
Certain filamentous fungi, such as the penicillin-producing strain Penicillium chrysogenum, secrete small, highly basic and cysteine-rich proteins with antifungal effects. Affected fungi include a number of important zoopathogens, including those infecting humans. Recent studies, however, have pointed to a membrane-perturbing effect of these antifungal compounds, apparent as a potassium efflux from affected fungal cells. If present on mammalian cells, this would severely hinder the potential therapeutic use of these molecules. Here we studied the effects of the P. chrysogenum-derived antifungal peptide (PAF) on a number of mammalian cells to establish whether the protein has any cytotoxic effects, alters transmembrane currents on excitable cells or activates the immune system. PAF, in a concentration range of 2–100 μg/ml, did not cause any cytotoxicity on human endothelial cells from the umbilical vein. Applied at 10 μg/ml, it also failed to modify voltage-gated potassium channels of neurones, skeletal muscle fibers, and astrocytes. PAF also left the hyperpolarization-activated non-specific cationic current (Ih) and the L-type calcium current unaffected. Finally, up to 2 μg/ml, PAF did not induce the production of pro-inflammatory cytokines such as IL-6, IL-8, and TNF-α. These results suggest that PAF should have only minor, if any, effects on mammalian cells in the intended therapeutic concentration range.






Similar content being viewed by others
References
Balla J, Jacob HS, Balla G, Nath K, Eaton JW, Vercelotti GM (1993) Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci USA 90:9285–9289
Barton GM, Medzhitov R (2002) Control of adaptive immune responses by toll-like receptors. Curr Opin Immunol 14:380–383
Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L (2004) The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol 172:3059–3069
Bers DM (2001) Excitation contraction coupling and cardiac contractile force. Kluwer Academic, Dordrecht
Braedel S, Radsak M, Einsele H, Latge JP, Michan A, Loeffler J, Haddad Z, Grigoleit U, Schild H, Hebart H (2004) Aspergillus fumigatus antigens activate innate immune cells via toll-like receptors 2 and 4. Br J Haematol 125:392–399
Catterall WA (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 16:521–555
Csernoch L, Szentesi P, Kovács L (1999) Differential effects of caffeine and perchlorate on excitation–contraction coupling in mammalian skeletal muscle. J Physiol (Lond) 520:217–230
Cuttle MF, Rusznak Z, Wong AY, Owens S, Forsythe ID (2001) Modulation of a presynaptic hyperpolarization-activated cationic current (Ih) at an excitatory synaptic terminal in the rat auditory brainstem. J Physiol (Lond) 534:733–744
Dascal N (2001) Ion-channel regulation by G proteins. Trends Endocrinol Metab 12:391–398
Dimarcq JL, Bulet P, Hetru C, Hoffmann J (1998) Cysteine-rich antimicrobial peptides in invertebrates. Biopolymers 47:465–477
Fritig B, Heitz T, Legrand M (1998) Antimicrobial proteins in induced plant defense. Curr Opin Immunol 10:16–22
Garcia-Olmedo F, Molina A, Alamillo JM, Rodriguez-Palenzuela P (1998) Plant defense peptides. Biopolymers 47:479–491
Geisen R (2000) P. nalgiovense carries a gene which is homologous to the paf gene of P. chrysogenum which codes for an antifungal peptide. Int J Food Microbiol 62:95–101
Goldin AL (1999) Diversity of mammalian voltage-gated sodium channels. In: Rudy B, Seeburg P (eds) Molecular and functional diversity of ion channels and receptors, vol 868. New York Academy of Sciences, New York, pp 38–50
Kaiserer L, Oberparleiter C, Weiler-Gorz R, Burgstaller W, Leiter E, Marx F (2003) Characterization of the Penicillium chrysogenum antifungal protein PAF. Arch Microbiol 180:204–210
Lakhani SA, Bogue CW (2003) Toll-like receptor signaling in sepsis. Curr Opin Pediatr 15:278–282
Lee DG, Shin SY, Maeng CY, Jin ZZ, Kim KL, Hahm KS (1999) Isolation and characterization of a novel antifungal peptide from Aspergillus niger. Biochem Biophys Res Commun 263:646–651
Lehrer RI, Ganz T (1996) Endogenous vertebrate antibiotics. Defensins, protegrins, and other cysteine-rich antimicrobial peptides. Ann NY Acad Sci 797:228–239
Lesage F, Lazdunski M (2000) Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol 279:F793–F801
Marx F (2004) Small, basic antifungal proteins secreted from filamentous ascomycetes: a comparative study regarding expression, structure, function and potential application. Appl Microbiol Biotechnol 65:133–142
Marx F, Haas H, Reindl M, Stoffler G, Lottspeich F, Redl B (1995) Cloning, structural organization and regulation of expression of the Penicillium chrysogenum paf gene encoding an abundantly secreted protein with antifungal activity. Gene 29:167–171
Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C (2003) Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci 23:1750–1758
McDonald TF, Pelzer S, Trautwein W, Pelzer DJ (1994) Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev 74:365–507
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
O’Neill LA (2002) Toll-like receptor signal transduction and the tailoring of innate immunity: a role for Mal? Trends Immunol 23:296–300
Ríos E, Pizarro G (1991) Voltage sensor of excitation–contraction coupling in skeletal muscle. Physiol Rev 71:849–908
Roberts SK (2003) TOK homologue in Neurospora crassa: first cloning and functional characterization of an ion channel in a filamentous fungus. Eukaryot Cell 2:181–190
Robinson RB, Siegelbaum SA (2003) Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65:453–480
Rusznák Z, Forsythe ID, Brew HM, Stanfield PR (1997) Membrane currents influencing action potential latency in granule neurons of the rat cochlear nucleus. Eur J Neurosci 9:2348–2358
Rusznák Z, Harasztosi C, Stanfield PR, Szűcs G (2001) An improved cell isolation technique for studying intracellular Ca2+ homeostasis in neurones of the cochlear nucleus. Brain Res Brain Res Protoc 7:68–75
Szentesi P, Jacquemond V, Kovács L, Csernoch L (1997) Intramembrane charge movement and sarcoplasmic calcium release in enzymatically isolated mammalian skeletal muscle fibers. J Physiol (Lond) 505:371–384
Szentesi P, Collet C, Sárközi S, Szegedi C, Jona I, Jacquemond V, Kovács L, Csernoch L (2001) Effects of dantrolene on steps of excitation–contraction coupling in mammalian skeletal muscle fibers. J Gen Physiol 118:355–375
Theis T, Stahl U (2004) Antifungal proteins: targets, mechanisms and prospective applications. Cell Mol Life Sci 61:437–455
Theis T, Wedde M, Meyer V, Stahl U (2003) The antifungal protein from Aspergillus giganteus causes membrane permeabilization. Antimicrob Agents Chemother 47:588–593
Triantafilou M, Triantafilou K (2002) Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol 23:301–304
Wnendt S, Ulbrich N, Stahl U (1994) Molecular cloning, sequence analysis and expression of the gene encoding an antifungal-protein from Aspergillus giganteus. Curr Genet 25:519–523
Acknowledgements
The authors wish to thank R. Öri and I. Varga for their technical assistance. The work was supported by research grants from OTKA (TS040773, T034894, T046067, T034315, and T037473) and the Office for Higher Education Programs (0092/2001) of Hungary. I.P. is a recipient of the Széchenyi István Scholarship. F.M. was supported by the Austrian Science Foundation (FWF grant P-15261) and the University of Innsbruck (grant X8).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szappanos, H., Szigeti, G.P., Pál, B. et al. The Penicillium chrysogenum-derived antifungal peptide shows no toxic effects on mammalian cells in the intended therapeutic concentration. Naunyn-Schmiedeberg's Arch Pharmacol 371, 122–132 (2005). https://doi.org/10.1007/s00210-004-1013-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-004-1013-7