Abstract.
The interface between receptors and G proteins can be considered as a drug target. Various classes of low molecular weight inhibitors have been identified that block the ability of receptors to interact with G proteins (e.g. peptides, suramin analogues and amphiphilic cations). Here we have tested if there are compounds that differentially affect the interaction of one receptor with two different (related) G protein α-subunits. Fusion proteins comprising the human A1-adenosine receptor and Gαi-1 (A1/Gαi-1) or Gαo (A1/Gαo) were expressed in HEK293 cells. Suramin analogues were screened for their ability to differentially affect high affinity binding of the agonist (-)N 6-3-[125I](iodo-4-hydroxyphenylisopropyl) adenosine (IHPIA).
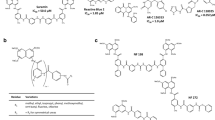
One compound [NF326 = 8,8'-(carbonylbis-(imino-3,1-phenylenecarbonylimino))bis-(1-naphthol-3,6-disulfonic acid, disodium salt)] was identified that inhibited high affinity agonist binding to the fusion protein A1/Gαi-1 but modestly enhanced binding of IHPIA to A1/Gαo. This action was specific because NF326 did not affect antagonist binding to either fusion protein. In addition, it was unrelated to a difference in affinity of the receptor for the G protein fusion moiety because the stability of ternary complexes formed by IHPIA + A1/Gαi-1 and IHPIA + A1/Gαo is comparable and because lowering the affinity of the receptor for the G protein (by introducing point mutations at cys351 of Gαi-1) enhanced the uncoupling effect of NF326. Finally, NF326 did not discriminate between a fusion protein comprising the α2A-adrenoceptor and Gαi-1 (α2A/Gαi-1) or Gαo-1 (α2A/Gαo-1); binding of the agonist [3H]UK14304 (bromoxidine) to both fusion proteins was inhibited over a comparable concentration range while binding of the antagonist [3H]yohimbine was unaffected.
These observations are consistent with the interpretation that the contact sites that are formed between individual receptors and G proteins differ. These differences suffice to allow for selective disruption by G protein inhibitors of different classes. Using NF326 we show that the bulk of the A1-adenosine receptors in human cerebrocortical membranes interacts with Gαo rather than Gαi.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Kudlacek, O., Waldhoer, M., Kassack, M.U. et al. Biased inhibition by a suramin analogue of A1-adenosine receptor/G protein coupling in fused receptor/G protein tandems: the A1-adenosine receptor is predominantly coupled to Goα in human brain. Naunyn-Schmied Arch Pharmacol 365, 8–16 (2002). https://doi.org/10.1007/s00210-001-0493-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00210-001-0493-y