Abstract
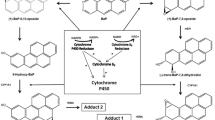
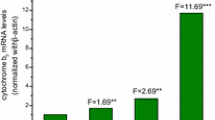
Benzene, a ubiquitous environmental pollutant, is haematotoxic and myelotoxic. As has been shown earlier, cytochrome P450 2E1 (CYP2E1)-dependent metabolism is a prerequisite for the cytotoxic and genotoxic effects of benzene, but which of the benzene metabolites produces toxicity is still unknown. The observed differences between the toxicity of benzene and that of phenol, a major metabolite of benzene, could be explained by alternative hypotheses. That is, whether (1) toxic benzene effects are caused by metabolites not derived from phenol (e.g. benzene epoxide, muconaldehyde), which are formed in the liver and are able to reach the target organ(s); or (2) benzene penetrates into the bone marrow, where local metabolism takes place, whereas phenol does not reach the target tissue because of its polarity. To further investigate hypothesis 2, we used various strains of mice (AKR, B6C3F1, CBA/Ca, CD-1 and C57Bl/6), for which different toxic responses have been reported in the haematopoietic system after chronic benzene exposure. In these strains, CYP2E1 expression in bone marrow was investigated and compared with CYP2E1 expression in liver by means of two independent methods. Quantification of CYP2E1-dependent hydroxylation of chlorzoxazone (CLX) by high-performance liquid chromatography (HPLC; functional analysis) was used to characterize specific enzymatic activities. Protein identification was performed by Western blotting using CYP2E1-specific antibodies. In liver microsomes of all strains investigated, considerable amounts of CYP2E1-specific protein and correspondingly high CYP2E1 hydroxylase activities could be detected. No significant differences in CYP2E1-dependent enzyme activities were found between the five strains (range of medians, 4.6–12.0 nmol 6-OH-CLX/[mg protein × min]) in hepatic tissue. In the bone marrow, CYP2E1 could also be detected in all strains investigated. However, chlorzoxazone hydroxylase activities were considerably lower (range of medians, 0.2–0.8 × 10−3 nmol 6-OH-CLX/[mg protein × min]) compared with those obtained from liver microsomes. No significant (P > 0.05) interstrain differences in CYP2E1 expression in liver and/or bone marrow could be observed in the mouse strains investigated. The data obtained thus far from our investigations suggest that strain-specific differences in the tumour response of the haematopoietic system of mice chronically exposed to benzene cannot be explained by differences in either hepatic or in myeloid CYP2E1-dependent metabolism of benzene.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 7 September 1998 / Accepted: 13 April 1999
Rights and permissions
About this article
Cite this article
Bernauer, U., Vieth, B., Ellrich, R. et al. CYP2E1-dependent benzene toxicity: the role of extrahepatic benzene metabolism. Arch Toxicol 73, 189–196 (1999). https://doi.org/10.1007/s002040050605
Issue Date:
DOI: https://doi.org/10.1007/s002040050605