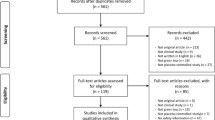
Abstract
Green tea (GT), obtained from the leaves of Camellia sinensis (L.) Kuntze (Fam. Theaceae), is largely used for its potential health benefits such as reduction in risk of cardiovascular diseases and weight loss. Nevertheless, it is suspected to induce liver damage. Present work reviews the hepatic adverse reactions associated with GT-based herbal supplements, published by the end of 2008 to March 2015. A systematic research was carried out on PubMed, MedlinePlus, Scopus and Google Scholar databases, without any language restriction. Moreover, some accessible databases on pharmacovigilance or phytovigilance were consulted. The causality assessment was performed using the CIOMS/RUCAM score. Nineteen cases of hepatotoxicity related to the consumption of herbal products containing GT were identified. The hepatic reactions involved mostly women (16/19); the kind of liver damage was generally classified as hepatocellular (16/19). The causality assessment between consumption of herbal preparation and hepatic reaction resulted as probable in eight cases and as possible in eleven cases. In seven cases, patients used preparations containing only GT, while twelve reactions involved patients who took multicomponent preparations (MC). The reactions induced by GT had a generally long latency (179.1 ± 58.95 days), and the outcome was always resolution, with recovery time of 64.6 ± 17.78 days. On the contrary, liver injury associated with MC had a shorter latency (44.7 ± 13.85 days) and was more serious in four cases that required liver transplantation and, when resolution occurred, the recovery time was longer (118.9 ± 38.79). MC preparations contained numerous other components, many of which are suspected to induce liver damage, so it is difficult to ascribe the toxicity to one specific component, e.g., GT. Present data confirm a certain safety concern with GT, even if the number of hepatic reactions reported is low considering the great extent of use of this supplement. The mechanism of GT hepatotoxicity remains unclear, but factors related to the patient are becoming predominant. A major safety concern exists when GT is associated with other ingredients that can interact between them and with GT, enhancing the risk of liver damage. Patients should be discouraged from using herbal or dietary supplements containing complex mixtures and should be encouraged to use herbal and dietary supplement possibly under supervision of healthcare professionals.
Similar content being viewed by others
References
Actis GC, Bugianesi E, Ottobrelli A, Rizzetto M (2007) Fatal liver failure following food supplements during chronic treatment with montelukast. Dig Liver Dis 39:953–955. doi:10.1016/j.dld.2006.10.002
Amariles P, Angulo N, Agudelo-Agudelo J, Gaviria G (2009) Hepatitis associated with aqueous green tea infusions: a case study. Farm Hosp 33:289–291. doi:10.1016/S1130-6343(09)72471-7
Anderson RA (1998) Effects of chromium on body composition and weight loss. Nutr Rev 56:266–270. doi:10.1111/j.1753-4887.1998.tb01763.x
Andrade RJ, Lucena MI, Fernández MC, Vega JL, García-Cortés M, Casado M, Guerrero-Sanchez E, Pulido-Fernandez F (2002) Cholestatic hepatitis related to use of irbesartan: a case report and a literature review of angiotensin II antagonist-associated hepatotoxicity. Eur J Gastroenterol Hepatol 14:887–890. doi:10.1097/00042737-200208000-00014
Arimone Y, Bégaud B, Miremont-Salamé G, Fourrier-Réglat A, Moore N, Molimard M, Haramburu F (2005) Agreement of expert judgment in causality assessment of adverse drug reactions. Eur J Clin Pharmacol 61:169–173. doi:10.1007/s00228-004-0869-2
Arroyo-Martinez Q, Sáenz MJ, Argüelles Arias F, Acosta MS (2011) Lycium barbarum: a new hepatotoxic “natural” agent? Dig Liver Dis 43:749. doi:10.1016/j.dld.2011.04.010
Arzenton E, Magro L, Paon V, Capra F, Apostoli P, Guzzo F, Conforti A, Leone R (2014) Acute epatitis caused by green tea infusion: a case report. Adv Pharmacoepidemiol Drug Saf 3:170. doi:10.4172/2167-1052.1000170
Bardia A, Nisly NL, Zimmerman MB, Gryzlak BM, Wallace RB (2007) Use of herbs among adults based on evidence-based indications: findings from the National Health Interview Survey. Mayo Clin Proc 82:561–566. doi:10.4065/82.5.561
Belfrage B, Malmström R (2008) Several cases of liver affected by Aloe vera. Lakartidningen 105:45
Benichou C, Danan G, Flahault A (1993) Causality assessment of adverse reactions to drugs–II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol 46:1331–1336. doi:10.1016/0895-4356(93)90102-7
Bergman J, Schjøtt J (2009) Hepatitis caused by Lotus-f3? Basic Clin Pharmacol Toxicol 104:414–416. doi:10.1111/j.1742-7843.2009.00385.x
Boehm K, Borrelli F, Ernst E, Habacher G, Hung SK, Milazzo S, Horneber M (2009) Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev 8:CD005004. doi:10.1002/14651858.CD005004.pub2
Bottenberg MM, Wall GC, Harvey RL, Habib S (2007) Oral Aloe vera-induced hepatitis. Ann Pharmacother 41:1740–1743. doi:10.1345/aph.1K132
Bunchorntavakul C, Reddy KR (2013) Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther 37:3–17. doi:10.1111/apt.12109
Butt MS, Ahmad RS, Sultan MT, Qayyum MM, Naz A (2015) Green tea and anticancer perspectives: updates from last decade. Crit Rev Food Sci Nutr 55:792–805. doi:10.1080/10408398.2012.680205
Cavaliere C, Rea C, Lynch ME, Blumenthal M (2010) Herbal supplement sales rise in all channels in 2009. HerbalGram 86:62–65
Cerulli J, Grabe DW, Gauthier I, Malone M, McGoldrick MD (1998) Chromium picolinate toxicity. Ann Pharmacother 32:428–431. doi:10.1345/aph.17327
Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J, Drug Induced Liver Injury Network (DILIN) (2008) Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 135: 1924–1934, 1934.e1–1934.e4. doi: 10.1053/j.gastro.2008.09.011
Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ (2014) ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 109:950–966. doi:10.1038/ajg.2014.131
Chang CH, Chang YC, Lee YC, Liu YC, Chuang LM, Lin JW (2015) Severe hepatic injury associated with different statins in patients with chronic liver disease: a nationwide population-based cohort study. J Gastroenterol Hepatol 30:155–162. doi:10.1111/jgh.12657
Chauhan B, Kumar G, Kalam N, Ansari SH (2013) Current concepts and prospects of herbal nutraceutical: a review. J Adv Pharm Technol Res 4:4–8. doi:10.4103/2231-4040.107494
Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS (2005) Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin Cancer Res 11:4627–4633. doi:10.1158/1078-0432.CCR-04-2549
Church RJ, Gatti DM, Urban TJ, Long N, Yang X, Shi Q, Eaddy JS, Mosedale M, Ballard S, Churchill GA, Navarro V, Watkins PB, Threadgill DW, Harrill AH (2015) Sensitivity to hepatotoxicity due to epigallocatechin gallate is affected by genetic background in diversity outbred mice. Food Chem Toxicol 76:19–26. doi:10.1016/j.fct.2014.11.008
Curciarello J, De Ortúzar S, Borzi S, Bosia D (2008) Severe acute hepatitis associated with intake of Aloe vera tea. Gastroenterol Hepatol 31:436–438. doi:10.1157/13125590
Danan G, Benichou C (1993) Causality assessment of adverse reactions to drugs-I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 46:1323–1330. doi:10.1016/0895-4356(93)90101-6
Dela Cruz AC, Patel TT, Maynard E, Shah M, Lee EY, Angulo P (2014) Fulminant liver failure secondary to “Saba appetite control and energy” weight loss supplement. Gastroenterology 146:S-1002. doi:10.1016/S0016-5085(14)63644-1
Denison HJ, Jameson KA, Syddall HE, Dennison EM, Cooper C, Sayer AA, Robinson SM (2012) Patterns of dietary supplement use among older men and women in the UK: findings from the Hertfordshire Cohort Study. J Nutr Health Aging 16:307–311. doi:10.1007/s12603-012-0016-1
Dias TR, Alves MG, Tomás GD, Socorro S, Silva BM, Oliveira PF (2014) White tea as a promising antioxidant medium additive for sperm storage at room temperature: a comparative study with green tea. J Agric Food Chem 62:608–617. doi:10.1021/jf4049462
Dong H, Slain D, Cheng J, Ma W, Liang W (2014) Eighteen cases of liver injury following ingestion of Polygonum multiflorum. Complement Ther Med 22:70–74. doi:10.1016/j.ctim.2013.12.008
Douros A, Kauffmann W, Bronder E, Klimpel A, Garbe E, Kreutz R (2013) Ramipril-induced liver injury: case report and review of the literature. Am J Hypertens 26:1070–1075. doi:10.1093/ajh/hpt090
Egras AM, Hamilton WR, Lenz TL, Monaghan MS (2011) An evidence-based review of fat modifying supplemental weight loss products. J Obes. doi:10.1155/2011/297315
Ehrenpreis ED, Kulkarni P, Burke C (2013) What gastroenterologists should know about the gray market, herbal remedies, and compounded pharmaceuticals and their regulation by the Food and Drug Administration. Am J Gastroenterol 108:642–646. doi:10.1038/ajg.2012.348
Elouni B, Ben Salem C, Zamy M, Ganne N, Beaugrand M, Bouraoui K, Biour M (2010) Cytolytic hepatitis possibly related to levonorgestrel/ethinylestradiol oral contraceptive use: 2 case reports. Ann Pharmacother 44:2035–2037. doi:10.1345/aph.1P201
Ernst E, De Smet PA, Shaw D, Murray V (1998) Traditional remedies and the “test of time”. Eur J Clin Pharmacol 54:99–100. doi:10.1007/s002280050428
Fan X, Chen P, Jiang Y, Wang Y, Tan H, Zeng H, Wang Y, Qu A, Gonzalez FJ, Huang M, Bi H (2015) Therapeutic efficacy of Wuzhi tablet (Schisandra sphenanthera extract) on acetaminophen-induced hepatotoxicity through a mechanism distinct from N-acetylcysteine. Drug Metab Dispos 43:317–324. doi:10.1124/dmd.114.062067
FDA (2013) DMAA in Dietary Supplements. US Food and Drug Administration. http://www.fda.gov/Food/DietarySupplements/QADietarySupplements/ucm346576.htm. Accessed 5 Mar 2015
FDA Warns Consumers to Stop Using Hydroxycut Products. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm149575.htm. Accessed 31 Mar 2015
Fernández J, Navascués C, Albines G, Franco L, Pipa M, Rodríguez M (2014) Three cases of liver toxicity with a dietary supplement intended to stop hair loss. Rev Esp Enferm Dig 106:552–555
Fong TL, Klontz KC, Canas-Coto A, Casper SJ, Durazo FA, Davern TJ II, Hayashi P, Lee WM, Seeff LB (2010) Hepatotoxicity due to hydroxycut: a case series. Am J Gastroenterol 105:1561–1566. doi:10.1038/ajg.2010.5
Foti RS, Dickmann LJ, Davis JA, Greene RJ, Hill JJ, Howard ML, Pearson JT, Rock DA, Tay JC, Wahlstrom JL, Slatter JG (2008) Metabolism and related human risk factors for hepatic damage by usnic acid containing nutritional supplements. Xenobiotica 38:264–280. doi:10.1080/00498250701802514
Franco M, Monmany J, Domingo P, Turbau M (2012) Autoimmune hepatitis triggered by consumption of Goji berries. Med Clin 139:320–321. doi:10.1016/j.medcli.2012.02.009
Frédérich M, Wauters JN, Tits M, Jason C, de Tullio P, Van der Heyden Y, Fan G, Angenot L (2011) Quality assessment of Polygonum cuspidatum and Polygonum multiflorum by 1H NMR metabolite fingerprinting and profiling analysis. Planta Med 77:81–86. doi:10.1055/s-0030-1250132
Gad AS, Khadrawy YA, El-Nekeety AA, Mohamed SR, Hassan NS, Abdel-Wahhab MA (2011) Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition 27:582–589. doi:10.1016/j.nut.2010.04.002
Galati G, Lin A, Sultan AM, O’Brien PJ (2006) Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med 40:570–580. doi:10.1016/j.freeradbiomed.2005.09.014
Gallo E, Maggini V, Berardi M, Pugi A, Notaro R, Talini G, Vannozzi G, Bagnoli S, Forte P, Mugelli A, Annese V, Firenzuoli F, Vannacci A (2013) Is green tea a potential trigger for autoimmune hepatitis? Phytomedicine 20:1186–1189. doi:10.1016/j.phymed.2013.07.004
Garcia-Alvarez A, Egan B, de Klein S, Dima L, Maggi FM, Isoniemi M, Ribas-Barba L, Raats MM, Meissner EM, Bade M, Bruno F, Salmenhaara M, Milà-Villarroel R, Knaze V, Hodgkins C, Marculescu A, Uusitalo L, Restani P, Serra-Majem L (2014) Usage of plant food supplements across six European countries: findings from the PlantLIBRA Consumer Survey. PLoS One 9:e92265. doi:10.1371/journal.pone.0092265
Ghabril M, Bonkovsky HL, Kum C, Davern T, Hayashi PH, Kleiner DE, Serrano J, Rochon J, Fontana RJ, Bonacini M, US Drug-Induced Liver Injury Network (2013) Liver injury from tumor necrosis factor-α antagonists: analysis of thirty-four cases. Clin Gastroenterol Hepatol 11(558–564):e3. doi:10.1016/j.cgh.2012.12.025
Gloro R, Hourmand-Ollivier I, Mosquet B, Mosquet L, Rousselot P, Salamé E, Piquet MA, Dao T (2005) Fulminant hepatitis during self-medication with hydroalcoholic extract of green tea. Eur J Gastroenterol Hepatol 17:1135–1137. doi:10.1097/00042737-200510000-00021
Graziadei IW, Joseph JJ, Wiesner RH, Therneau TM, Batts KP, Porayko MK (1998) Increased risk of chronic liver failure in adults with heterozygous alpha1-antitrypsin deficiency. Hepatol 28:1058–1063. doi:10.1002/hep.510280421
Grube B, Chong PW, Lau KZ, Orzechowski HD (2013) A natural fiber complex reduces body weight in the overweight and obese: a double-blind, randomized, placebo-controlled study. Obesity 21:58–64. doi:10.1002/oby.20244
Guo L, Shi Q, Fang JL, Mei N, Ali AA, Lewis SM, Leakey JE, Frankos VH (2008) Review of usnic acid and Usnea barbata toxicity. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 26:317–338. doi:10.1080/10590500802533392
Gürgen S, Yücel A, Karakuş A, Ceçen D, Ozen G, Koçtürk S (2014) Usage of whey protein may cause liver damage via inflammatory and apoptotic responses. Hum Exp Toxicol. doi:10.1177/0960327114556787
Haimowitz S, Hsieh J, Shcherba M, Averbukh Y (2015) Liver failure after hydroxycut™ use in a patient with undiagnosed hereditary coproporphyria. J Gen Intern Med. doi:10.1007/s11606-014-3153-x
Hayashi PH, Barnhart HX, Fontana RJ, Chalasani N, Davern TJ, Talwalkar JA, Reddy KR, Stolz AA, Hoofnagle JH, Rockey DC (2014) Reliability of causality assessment for drug, herbal and dietary supplement hepatotoxicity in the Drug-Induced Liver Injury Network (DILIN). Liver Int. doi:10.1111/liv.12540
Huang L, Zhao H, Huang B, Zheng C, Peng W, Qin L (2011) Acanthopanax senticosus: review of botany, chemistry and pharmacology. Pharmazie 66:83–97. doi:10.1691/ph.2011.0744
Inno A, Basso M, Vecchio FM, Marsico VA, Cerchiaro E, D’Argento E, Bagalà C, Barone C (2011) Anastrozole-related acute hepatitis with autoimmune features: a case report. BMC Gastroenterol 11:32. doi:10.1186/1471-230X-11-32
Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J (2006) Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: dermal, acute and short-term toxicity studies. Food Chem Toxicol 44:636–650. doi:10.1691/ph.2011.0744
Jiménez-Encarnación E, Ríos G, Muñoz-Mirabal A, Vilá LM (2012) Euforia-induced acute hepatitis in a patient with scleroderma. BMJ Case Rep. doi:10.1136/bcr-2012-006907
Jimenez-Saenz M, Martinez-Sanchez C (2007) Green tea extracts and acute liver failure: the need for caution in their use and diagnostic assessment. Liver Transpl 13:1067. doi:10.1002/lt.21127
Jodoin J, Demeule M, Beliveau R (2002) Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim Biophys Acta 1542:149–159. doi:10.1016/S0167-4889(01)00175-6
Kågedal K, Bironaite D, Ollinger K (1999) Anthraquinone cytotoxicity and apoptosis in primary cultures of rat hepatocytes. Free Radic Res 31:419–428. doi:10.1080/10715769900300981
Kanat O, Ozet A, Ataergin S (2006) Aloe vera-induced acute toxic hepatitis in a healthy young man. Eur J Intern Med. 17:589. doi:10.1016/j.ejim.2006.04.017
Kaswala D, Shah S, Patel N, Raisoni S, Swaminathan S (2014) Hydroxycut-induced liver toxicity. Ann Med Health Sci Res 4:143–145. doi:10.4103/2141-9248.126627
Keske MA, Ng HL, Premilovac D, Rattigan S, Kim JA, Munir K, Yang P, Quon MJ (2015) Vascular and metabolic actions of the green tea polyphenol epigallocatechin gallate. Curr Med Chem 22:59–69. doi:10.2174/0929867321666141012174553
Kim YJ, Choi MS, Park YB, Kim SR, Lee MK, Jung UJ (2013) Garcinia cambogia attenuates diet-induced adiposity but exacerbates hepatic collagen accumulation and inflammation. World J Gastroenterol 19:4689–4701. doi:10.3748/wjg.v19.i29.4689
Koncic MZ, Tomczyk M (2013) New insights into dietary supplements used in sport: active substances, pharmacological and side effects. Curr Drug Targets 4:1079–1092. doi:10.2174/1389450111314090016
Lacey R, Evans A (2014) An unusual cause of jaundice in a patient with breast cancer. BMJ Case Rep. doi:10.1136/bcr-2014-205764
Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS (2010) Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food Chem Toxicol 48:409–416. doi:10.1016/j.fct.2009.10.030
Lança S, Alves A, Vieira AI, Barata J, de Freitas J, de Carvalho A (2002) Chromium-induced toxic hepatitis. Eur J Intern Med 13:518–520. doi:10.1016/S0953-6205(02)00164-4
Lee J, Lee MS, Nam KW (2014) Acute toxic hepatitis caused by an Aloe vera preparation in a young patient: a case report with a literature review. Korean J Gastroenterol 64:54–58. doi:10.4166/kjg.2014.64.1.54
Lei X, Chen J, Ren J, Li Y, Zhai J, Mu W, Zhang L, Zheng W, Tian G, Shang H (2015) Liver damage associated with polygonum multiflorum thunb: a systematic review of case reports and case series. Evid Based Complement Altern Med. doi:10.1155/2015/459749
Licata A, Macaluso FS, Craxì A (2013) Herbal hepatotoxicity: a hidden epidemic. Intern Emerg Med 8:13–22. doi:10.1007/s11739-012-0777-x
López-Cepero Andrada JM, Lerma Castilla S, Fernández Olvera MD, Amaya Vidal A (2007) Hepatotoxicity caused by a Noni (Morinda citrifolia) preparation. Rev Esp Enferm Dig 99:179–181
Lorenzo-Almorós A, Polo-Sabau J, Barrio-Dorado MD, Ruggiero García M (2015) Acute liver injury induced by green tea extracts. Gastroenterol Hepatol 38:44–45. doi:10.1016/j.gastrohep.2014.07.004
Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S (2009) Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol 65:331–341. doi:10.1007/s00228-008-0610-7
Mc Donnell WM, Bhattacharya R, Halldorson JB (2009) Fulminant hepatic failure after use of the herbal weight-loss supplement Exilis. Ann Intern Med 151:673–674. doi:10.7326/0003-4819-151-9-200911030-00021
Menniti-Ippolito F, Mazzanti G, Vitalone A, Firenzuoli F, Santuccio C (2008) Surveillance of suspected adverse reactions to natural health products: the case of propolis. Drug Saf 31:419–423. doi:10.2165/00002018-200831050-00007
Millonig G, Stadlmann S, Vogel W (2005) Herbal hepatotoxicity: acute hepatitis caused by a Noni preparation (Morinda citrifolia). Eur J Gastroenterol Hepatol 17:445–447
Moreira CT, Oliveira AL, Comar JF, Peralta RM, Bracht A (2013) Harmful effects of usnic acid on hepatic metabolism. Chem Biol Interact 203:502–511. doi:10.1016/j.cbi.2013.02.001
Mrzljak A, Kosuta I, Skrtic A, Kanizaj TF, Vrhovac R (2013) Drug-induced liver injury associated with Noni (Morinda citrifolia) juice and phenobarbital. Case Rep Gastroenterol 7:19–24. doi:10.1159/000343651
Neff GW, Reddy KR, Durazo FA, Meyer D, Marrero R, Kaplowitz N (2004) Severe hepatotoxicity associated with the use of weight loss diet supplements containing ma huang or usnic acid. J Hepatol 41:1062–1064. doi:10.1016/j.jhep.2004.06.028
Nortadas R, Barata J (2012) Fulminant hepatitis during self-medication with conjugated linoleic acid. Ann Hepatol 11:265–267
Patel SS, Beer S, Kearney DL, Phillips G, Carter BA (2013) Green tea extract: a potential cause of acute liver failure. World J Gastroenterol 19:5174–5177. doi:10.3748/wjg.v19.i31.5174
Perdices EV, Medina-Cáliz I, Hernando S, Ortega A, Martín-Ocaña F, Navarro JM, Peláez G, Castiella A, Hallal H, Romero-Gómez M, González-Jiménez A, Robles-Díaz M, Lucena MI, Andrade RJ (2014) Hepatotoxicity associated with statin use: analysis of the cases included in the Spanish Hepatotoxicity Registry. Rev Esp Enferm Dig 106:246–254
Pillukat MH, Bester C, Hensel A, Lechtenberg M, Petereit F, Beckebaum S, Müller KM, Schmidt HH (2014) Concentrated green tea extract induces severe acute hepatitis in a 63-year-old woman—a case report with pharmaceutical analysis. J Ethnopharmacol 155:165–170. doi:10.1016/j.jep.2014.05.015
Powers SK, Jackson MJ (2008) Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88(4):1243–1276. doi:10.1152/physrev.00031.2007
Radha Krishna Y, Mittal V, Grewal P, Fiel MI, Schiano T (2011) Acute liver failure caused by ‘fat burners’ and dietary supplements: a case report and literature review. Can J Gastroenterol 25:157–160
Ramos R, Mascarenhas J, Duarte P, Vicente C, Casteleiro C (2009) Conjugated linoleic acid-induced toxic hepatitis: first case report. Dig Dis Sci 54:1141–1143. doi:10.1007/s10620-008-0461-1
RELIS database (2007) CLA, Lotus-f3 og hepatitt. RELIS Vest. http://relis.arnett.no/Utredning_Ekstern.aspx?Relis=3&S=4390&R=X. Accessed 11 Mar 2015
Rietjens IM, Boersma MG, Haan Ld, Spenkelink B, Awad HM, Cnubben NH, van Zanden JJ, Hv Woude, Alink GM, Koeman JH (2002) The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ Toxicol Pharmacol 11(3–4):321–333
Rietveld A, Wiseman S (2003) Antioxidant effects of tea: evidence from human clinical trials. J Nutr 133:3285S–3292S
Rohde J, Jacobsen C, Kromann-Andersen H (2011) Toxic hepatitis triggered by green tea. Ugeskr Laeger 173:205–206
Rossi S, Navarro VJ (2014) Herbs and liver injury: a clinical perspective. Clin Gastroenterol Hepatol 12:1069–1076. doi:10.1016/j.cgh.2013.07.030
Sanchez W, Maple JT, Burgart LJ, Kamath PS (2006) Severe hepatotoxicity associated with use of a dietary supplement containing usnic acid. Mayo Clin Proc 81:541–544. doi:10.4065/81.4.541
Sarma DN, Barrett ML, Chavez ML, Gardiner P, Ko R, Mahady GB, Marles RJ, Pellicore LS, Giancaspro GI, Low Dog T (2008) Safety of green tea extracts: a systematic review by the US Pharmacopeia. Drug Saf 31:469–484. doi:10.2165/00002018-200831060-00003
Schönthal AH (2011) Adverse effects of concentrated green tea extracts. Mol Nutr Food Res 55:874–885. doi:10.1002/mnfr.201000644
Seeff LB, Bonkovsky HL, Navarro VJ, Wang G (2014) Herbal products and the liver: a review of adverse effects and mechanisms. Gastroenterology. doi:10.1053/j.gastro.2014.12.004
Sharma T, Wong L, Tsai N, Wong RD (2010) Hydroxycut(®) (herbal weight loss supplement) induced hepatotoxicity: a case report and review of literature. Hawaii Med J 69:188–190
Shiyovich A, Sztarkier I, Nesher L (2010) Toxic hepatitis induced by Gymnema sylvestre, a natural remedy for type 2 diabetes mellitus. Am J Med Sci 340:514–517. doi:10.1097/MAJ.0b013e3181f41168
Silano V, Coppens P, Larrañaga-Guetaria A, Minghetti P, Roth-Ehrang R (2011) Regulations applicable to plant food supplements and related products in the European Union. Food Funct 2:710–719. doi:10.1039/c1fo10105f
Snyder FJ, Dundas ML, Kirkpatrick C, Neill KS (2009) Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nutr Elder 28:81–95. doi:10.1080/01639360802634043
Stadlbauer V, Fickert P, Lackner C, Schmerlaib J, Krisper P, Trauner M, Stauber RE (2005) Hepatotoxicity of NONI juice: report of two cases. World J Gastroenterol 11:4758–4760
Stadlbauer V, Weiss S, Payer F, Stauber RE (2008) Herbal does not at all mean innocuous: the sixth case of hepatotoxicity associated with Morinda citrifolia (Noni). Am J Gastroenterol 103:2406–2407. doi:10.1111/j.1572-0241.2008.02010_8.x
Stickel F, Kessebohm K, Weimann R, Seitz HK (2011) Review of liver injury associated with dietary supplements. Liver Int 31:595–605. doi:10.1111/j.1478-3231.2010.02439.x
Tenore GC, Daglia M, Ciampaglia R, Novellino E (2015) Exploring the nutraceutical potential of polyphenols from black, green and white tea infusions—an overview. Curr Pharm Biotechnol 16:264–271. doi:10.2174/1389201016666150118133604
Teschke R, Schwarzenboeck A, Hennermann KH (2008) Causality assessment in hepatotoxicity by drugs and dietary supplements. Br J Clin Pharmacol 66:758–766. doi:10.1111/j.1365-2125.2008.03264.x
Teschke R, Zhang L, Melzer L, Schulze J, Eickhoff A (2014) Green tea extract and the risk of drug-induced liver injury. Expert Opin Drug Metab Toxicol 10:1663–1676. doi:10.1517/17425255.2014.971011
Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard-Caudron AS, Pineau B, Weber P (2003) A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res 31:88–101
Update on the USP Green Tea Extract Monograph. http://www.usp.org/usp-nf/notices/retired-compendial-notices/update-usp-green-tea-extract-monograph. Accessed 31 Mar 2015
Urban TJ, Daly AK, Aithal GP (2014) Genetic basis of drug-induced liver injury: present and future. Semin Liver Dis 34:123–133. doi:10.1055/s-0034-1375954
US National Library of Medicine—NIH http://www.nlm.nih.gov/medlineplus/healthtopics.html. Accessed 16 Feb 2015 (bisogna indicare il documento consultato)
Vanstraelen S, Rahier J, Geubel AP (2008) Jaundice as a misadventure of a green tea (Camellia sinensis) lover: a case report. Acta Gastroenterol Belg 71:409–412
Verhelst X, Burvenich P, Van Sassenbroeck D, Gabriel C, Lootens M, Baert D (2009) Acute hepatitis after treatment for hair loss with oral green tea extracts (Camellia sinensis). Acta Gastroenterol Belg 72:262–264
Vilella AL, Limsuwat C, Williams DR, Seifert CF (2013) Cholestatic jaundice as a result of combination designer supplement ingestion. Ann Pharmacother 47:e33. doi:10.1345/aph.1R405
Villalta D, Imbastaro T, Di Giovanni S, Lauriti C, Gabini M, Turi MC, Bizzaro N (2012) Diagnostic accuracy and predictive value of extended autoantibody profile in systemic sclerosis. Autoimmun Rev 12:114–120. doi:10.1016/j.autrev.2012.07.005
Vitalone A, Menniti-Ippolito F, Moro PA, Firenzuoli F, Raschetti R, Mazzanti G (2011) Suspected adverse reactions associated with herbal products used for weight loss: a case series reported to the Italian National Institute of Health. Eur J Clin Pharmacol 67:215–224. doi:10.1007/s00228-010-0981-4
Vitalone A, Menniti-Ippolito F, Raschetti R, Renda F, Tartaglia L, Mazzanti G (2012) Surveillance of suspected adverse reactions to herbal products used as laxatives. Eur J Clin Pharmacol 8:231–238. doi:10.1007/s00228-011-1128-y
Waldman W, Piotrowicz G, Sein Anand J (2013) Hepatoxic effect of a noni juice consumption—a case report. Przegl Lek 70:690–692
Wang L, Zhang X, Liu J, Shen L, Li Z (2014) Tea consumption and lung cancer risk: a meta-analysis of case–control and cohort studies. Nutrition 30:1122–1127. doi:10.1016/j.nut.2014.02.023
Weinstein DH, Twaddell WS, Raufman JP, Philosophe B, Mindikoglu AL (2012) SlimQuick™-associated hepatotoxicity in a woman with alpha-1 antitrypsin heterozygosity. World J Hepatol 4:154–157. doi:10.4254/wjh.v4.i4.154
West BJ, Deng S (2011) Ingredients other than noni may be culprits in acute hepatotoxicity in 14-year-old boy. J Pediatr Gastroenterol Nutr 53:469–470. doi:10.1097/MPG.0b013e31822b77e2
Whitsett M, Halegoua-De Marzio D, Rossi S (2014) SlimQuick™-associated hepatotoxicity resulting in fulminant liver failure and orthotopic liver transplantation. ACG Case Rep J 1:220–222
Whitt KN, Ward SC, Deniz K, Liu L, Odin JA, Qin L (2008) Cholestatic liver injury associated with whey protein and creatine supplements. Semin Liver Dis 28:226–231. doi:10.1055/s-2008-1073122
Wierzejska R (2014) Tea and health—a review of the current state of knowledge. Przegl Epidemiol 68(501–6):595–599
Wu KM, Yao J, Boring D (2001) Green tea extract-induced lethal toxicity in fasted but not in nonfasted dogs. Int J Toxicol 30:19–20. doi:10.1177/1091581810387445
Wu QJ, Dong QH, Sun WJ, Huang Y, Wang QQ, Zhou WL (2014) Discrimination of Chinese teas with different fermentation degrees by stepwise linear discriminant analysis (S-LDA) of the chemical compounds. J Agric Food Chem 62:9336–9344. doi:10.1021/jf5025483
Yang HN, Kim DJ, Kim YM, Kim BH, Sohn KM, Choi MJ, Choi YH (2010) Aloe-induced toxic hepatitis. J Korean Med Sci 25:492–495. doi:10.3346/jkms.2010.25.3.492
Yu EL, Sivagnanam M, Ellis L, Huang JS (2011) Acute hepatotoxicity after ingestion of Morinda citrifolia (Noni Berry) juice in a 14-year-old boy. J Pediatr Gastroenterol Nutr 52:222–224. doi:10.1097/MPG.0b013e3181eb69f0
Yuce B, Gulberg V, Diebold J, Gerbes AL (2006) Hepatitis induced by Noni juice from Morinda citrifolia: a rare cause of hepatotoxicity or the tip of the iceberg? Digestion 73:167–170
Zhou S, Lim LY, Chowbay B (2004) Herbal modulation of P-glycoprotein. Drug Metab Rev 36:57–104. doi:10.1081/DMR-120028427
Acknowledgments
A. Di Sotto and A. Vitalone were supported by “Enrico and Enrica Sovena Foundation” Rome, Italy. We thank Dr. Patrizia Parisi and Dr. Silvia Di Giacomo for their assistance in retrieving some articles.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Antonella Di Sotto and Annabella Vitalone have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Mazzanti, G., Di Sotto, A. & Vitalone, A. Hepatotoxicity of green tea: an update. Arch Toxicol 89, 1175–1191 (2015). https://doi.org/10.1007/s00204-015-1521-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1521-x