Abstract
Acidovorax citrulli, the gram-negative bacteria that causes bacterial fruit blotch (BFB), has been responsible for huge worldwide economic losses in watermelon and melon production since 1980. No commercial cultivar resistant to BFB has been reported. Of the two reported genotypes of A. citrulli, genotype I is the main causal agent of BFB in melon and genotype II causes disease in watermelon. After the isolation of the first bacteriophage against A. citrulli (ACP17), efforts have been made to isolate bacteriophages with wider host ranges by collecting samples from watermelon, pumpkin, and cucumber. The newly isolated phage ACPWH, belonging to the Siphoviridae family, has a head size of 60 ± 5 nm and tail size of 180 ± 5 nm, and can infect 39 out of 42 A. citrulli strains. ACPWH has genome size of 42,499 and GC content of 64.44%. Coating watermelon seeds with bacteriophage ACPWH before soil inoculation with A. citrulli resulted in 96% germination and survival, compared to 13% germination of uncoated control seeds. These results suggest that phage ACPWH may be an effective and low-cost biocontrol agent against BFB.
Similar content being viewed by others
Introduction
Li Watermelon (Citrullus lanatus) belongs to the Cucurbitaceae family, along with other popular fruits such as melon (Cucumis melo), cucumber (Cucumis sativus), and pumpkin (Cucurbita pepo). Citrullus lanatus is among many widely cultivated vegetable crops that originated in Africa and spread throughout the world (Guo et al. 2012). According to data from the Food and Agriculture Organization of the United Nations (Databases | FAO | Food and Agriculture Organization of the United Nations 2016) total watermelon production in four major watermelon producing countries, China, the United States, Turkey, and Iran, was 88 million tons in 2016, which is more than 50% of the total global watermelon production that year. Watermelon fruit contains vitamins (A, B, and E) and essential minerals such as calcium, magnesium, and iron. It also contains antioxidants and the amino acid citrulline, which makes watermelon an important fruit in the human diet (Tlili et al. 2011). Studies have shown that watermelon not only has economic importance, but also that consumption of this fruit can promote proper blood circulation (Curis et al. 2005), control cardiovascular diseases, and reduce pulmonary hypertension (Smith et al. 2006).
Bacterial fruit blotch (BFB), caused by the gram-negative phytobacteria Acidovorax citrulli, was first reported in watermelon fields in the United States in the late 1980s, and has spread worldwide. Since then, BFB has been reported as a pathogen in other cucurbits such as pumpkin, melon, honeydew, and cucumber (Burdman and Walcott 2012a, b). Seed-borne bacterial pathogens are known passive carriers of plant diseases such as BFB (Hopkins et al. 2003) which can occur at any stage of plant growth. Symptoms of BFB include water-soaked seedlings and light brown-reddish colored lesions on the leaves. In addition, small water-soaked regions on a fruit surface can crack the surface; this can lead to total fruit loss, resulting in huge economic losses (Tian et al. 2015).
The host–pathogen relationship between watermelon and A. citrulli was shown to be highly related to Type IV pili and polar flagella, which play roles in twitching motility and biofilm formation (Johnson et al. 2011). The type III secretion system (T3SS) is another factor in the pathogenicity of A. citrulli, followed by the hypersensitive response in the plant. T3SS-encoding genes are also known as hypersensitive response genes, and mutations of these genes can result in non-pathogenic A. citrulli (Eckshtain-Levi et al. 2014). Genetic analysis and DNA fingerprinting by pulse-field gel electrophoresis showed that A. citrulli populations can be divided into three districts groups (Zivanovic and Walcott 2016). Group 1 strains are mainly isolated from non-watermelon hosts and are virulent in several cucurbit hosts. The group 2 strains cause virulent disease in watermelon. The third group comprises two strains of A. citrulli, ZUM4000 and ZUM4001, which cause weak disease in cucurbits (Eckshtain-Levi et al. 2014).
Many studies have been conducted, and efforts have been made, to control cucurbit diseases, but A. citrulli remains a threat to commercial cucurbit production. Walcott et al. (2004) reported that strategies such as the use of copper-based compounds were unsuccessful in controlling the disease; thus, integrated disease management strategies are employed but remain ineffective for eradicating BFB. Compared to other available strategies for plant bacterial diseases, seed treatment is a low-cost control method, and combinations of various seed treatment techniques have been recommended to produce A. citrulli-free seeds (Sharma et al. 2015). Most available studies suggest that A. citrulli is undamaged by antimicrobial treatment due to its presence in the seed coat and embryo. Currently available chemical compounds, such as sodium hypochlorite and hydrochloric acid (HC1), can effectively reduce BFB incidence but due to the location of A. citrulli, complete eradication of the pathogen has not yet occurred. Nevertheless, seed fermentation, seed washing, and seed drying, which are critical strategies to reduce seed-transmitted diseases, fail to completely control BFB (Burdman and Walcott 2012a, b).
Viruses infecting bacteria, called bacteriophages (phages), were identified for the first time by Twort and d’Herelle (Mukerjee 1963). Phages are the most common entity on the planet and can only infect specific genera of bacteria, which makes bacteriophages candidate biocontrol agents for bacterial diseases. A few years after the discovery of phages, scientists aimed to use them for controlling bacterial diseases (Duckworth 1976), particularly in croplands, as phage-mediated biocontrol (Callanan and Klaenhammer 2008). The isolation and application of phages against Ralstonia solanacearum (Yamada 2013) and Pseudomonas phaseolicola (Hassan and El-Meneisy 2014) demonstrated the ability of phages to control diseases. Bacteriophage applications show advantages over other strategies, and the emergence of agrophage-producing companies underscores that phages are promising disease control agents. However, due to excessive antibiotic use, phage biocontrol has not yet been investigated or developed for many pathogenic bacteria, such as A. citrulli. In addition, available treatments have not shown protection after germination for BFB symptoms which is shown to be destructive after germination. Hence, in a previous study, we isolated a bacteriophage that infects A. citrulli for the first time. Although this phage, named ACP17, showed protective effects against BFB when seeds were coated with phage, it showed a limited host range for group I A. citrulli strains. Therefore, we searched for bacteriophages with wider host ranges for both group I and II strains, and here we aim to isolate a phage with wider host range and investigate the effect of phage coat on watermelon plant in presence of A. citrulli.
Material and methods
Strains and culture conditions
Bacteria strains were kindly provided by Professor Chang-Sik Oh of Kyung Hee University, Korea (Table 1), and were grouped by polymerase chain reaction (PCR) assay results using the group 2-specific forward primer G2AcFwd (CGATAGGGTTGGGTTCAAG), the group I and II common forward primer G12AcFwd (CCGAAGAGATAACACTGCATC), and the group I and II common reverse primer G12AcRev (ACGTACTGCCGATTTTTGC) (Zivanovic and Walcott 2016). All A. citrulli strains were grown in King’s B (KB) broth and KB agar overnight at 37 °C.
Phage isolation
Samples of soil, leaves, and stems of watermelon, pumpkin, and cucumber from 43 sampling sites (mostly watermelon farms) were collected. Ten grams of collected sample was washed with 10 mL of distilled water for 10 min, filtered using 0.2 μm filters (Minisart), and then stored at 4 °C until further analysis. Phages in the samples were enriched by adding 5 mL of group I or group II host cocktail to 5 mL fresh KB media and then mixed with 10 mL of prepared sample, followed by incubation at 37 °C for 48 h. To make a cocktail of A. citrulli strains, single colonies of the 25 group I strains and 17 group II strains listed in Table 1 were grown separately in 20 mL of fresh media overnight, and then 10 mL of overnight liquid culture was added to 200 mL fresh KB media in two separate flasks (group I cocktail and group II cocktail) and incubated for 5 h at 37 °C.
After incubation, cultured lysates were prepared by filtering through 0.2 μm filters, and the presence of phages was determined by spot assay, as follows: 300 μL of overnight culture of A. citrulli strains (Table 1) was separately added to 4 mL of top agar (0.7 agar), poured on a KB plate, and allowed to solidify for 10 min. Enriched culture lysate (3 μL) was spotted on the bacterial lawn and incubated overnight at 37 °C. Bacteriophages were isolated from positive samples using plaque-forming assays and plaque pick up method. Plaques were picked up using sterile Pasteur pipets and added to 1 mL SM buffer (100 mM NaCl, 8 mM MgSO4•7H2O, 50 mM Tris–HCl [pH 7.5]). Plaque assays and plaque pick-up were repeated three times to isolate pure bacteriophages.
Host range analysis
Bacteriophage infectivity was tested against 42 A. citrulli strains and 40 other bacterial strains, as listed in Supplementary Table 1. Each bacterium was grown overnight, and 300 μL of bacterial culture was mixed with 4 mL KB soft agar and overlaid on KB agar plates. Phages (5 μL) were spotted onto the plates and incubated overnight at 37 °C. A broad host range bacteriophage named ACPWH was chosen for further characterization. The infectivity of ACPWH against other plant pathogenic bacteria was assessed using spot assays and plaque assay, as described previously (Czajkowski et al. 2014).
Electron microscopy analysis
ACPWH lysate (20 mL) was concentrated by ultracentrifugation at 17,000×g for 4 h. Pellets were resuspended in 100 μL phosphate-buffered saline (PBS), titered and adjusted to 109 plaque-forming units (PFUmL−1), and stored at 4 °C. Phages in this concentrated lysate were negatively stained with 1% uranyl acetate and analyzed using transmission electron microscopy (TEM; Hitachi H-7500; Hitachi Ltd., Tokyo, Japan) at Pukyong National University, Busan, South Korea. Bacteriophage sizes were measured using ImageJ software (NIH, Bethesda, MD, USA).
Phage propagation and genome extraction
ACPWH was propagated using the top agar method, as described previously (Bonilla et al. 2016) with minor modifications. Acidovorax citrulli strain KACC17000 was grown to exponential phase; phage was then added to a final multiplicity of infection (MOI) of 0.01 and mixed with 5 mL KB soft agar, followed by incubation at 37 °C overnight. Virus particles were obtained by adding 5 mL SM buffer to the plate and agitating for 30 min. SM buffer was collected and centrifuged at 3000×g for 20 min at 4 °C to remove bacterial and agar residues. Purification of bacteriophages was performed by adding polyethylene glycol (PEG) 6000 to a final concentration of 10% (w/v) and stirred at 4 °C overnight. Bacteriophages were collected by centrifugation at 24,000×g at 4 °C for 1 h. Pelleted phage was resuspended in PBS and the titer was determined by plaque assay and stored at 4 °C until further use.
Two ml of Phage stock (1010 PFU/mL) was treated with 0.5 µL of DNase (1U/µl) and 1µL of Rnase A (10 mg/mL) at 37 °C for 30 min. Enzymes were deactivated using proteinase K and incubated at 60 °C for an hour. Phage DNA extraction was done using phenol: chloroform: isoamyl alcohol (PCI) and ethanol precipitation provided as previously mentioned (Sambrook and Russell 2006). Extracted genome was treated with 0.5 µL of DNAs (1U/µL), 1 µL of RNase (10 mg/mL) and 2 µL exonuclease III (50 µL/mL units) for the characterization of the genome.
Sequencing of viral genome
Genomic library and sequence analysis were performed by Teragene Korea Co., Seoul. Purified genomic DNA was randomly sheared to yield DNA fragments of average 350 bp in size using a Covaris S2 Ultrasonicator. Library preparation was performed following the Illumina TruSeq DNA PCR-free preparation Kit. Adaptor enrichments were performed using PCR according to the manufacturer’s instructions. The final library size and quality were evaluated electrophoretically with an Agilent High Sensitivity DNA Kit. The 150 bp paired-end reads were sequenced on Illumina HiSeq 4000 platform. Further image analysis and base calling were performed with RTA 2.7.3 (Real-Time Analysis) and bcl2fastq v2.17.1.14.
Short-reads were reduced using digital normalization with khmer v.0.8.4 for improve assembly. Normalized reads were assembled using IDBA-UD ver 1.1.1. Contigs were aligned to NCBI nt database using BLASTN with E value cutoff of 1e−10. Alignment results were classified taxonomy using Krona tools. Bacteriophage contigs were extracted using in-house script. Gene models were predicted using GeneMark.hmm with Heuristic models.
Phylogenetic tree of ACPWH was constructed comparing major capsid protein of bacteriophages belonging to Siphoviridae, Podoviridae and Myoviridae family. Maximum likelihood tree was drawn by multiple sequence alignment and neighbor joining method using mega 7 (Kumar et al. 2016).
One-step growth analysis
A one-step growth experiment was performed in bacteriophage ACPWH using previously described methods with some modifications (Ellis and Delbrück 1939). Briefly, 1 mL of ACPWH was added to 50 mL exponential phase A. citrulli strain KACC17000 at a MOI of 0.01 and left for 10 min at room temperature. The mixture was centrifuged at 3500×g for 30 min, and unabsorbed phage in the supernatant was discarded. The pellet was resuspended in 50 mL fresh KB media and incubated at 37 °C. Samples were collected at 10-min intervals, followed by filtration and plaque assays to calculate the number of phages.
pH stability and thermal stability
Bacteriophage ACPWH was prepared in 1 M Tris–HCl to a final concentration of 107 PFU mL−1. Phage suspension was incubated at pH 2, 5, 5.5, 6, 7, 8, 9, 10, 11, and 12. Phage prepared at pH 7 was also incubated at − 80 °C, − 20 °C, 4 °C, 15 °C, 25 °C, 37 °C, 45 °C, 50 °C, 55 °C, and 60 °C. Phage survival was measured every 12 h for 3 days with three replications of plaque assays, as described above.
In planta assay
Commercial watermelon seeds were washed with 1% sodium hypochlorite for 20 min to remove bacterial contamination and artificial seed colorant, then rinsed three times with sterilized water. After washing, seed coating was carried out by mixing seeds in suspensions of phage or bacteria separately for 20 min, then drying. Phage-coated seeds (n = 50) were prepared by mixing seeds in SM buffer containing 106 PFU mL−1 phage ACPWH and bacteria coated seeds (n = 50) were mixed with PBS buffer containing 105 colony forming units (CFU mL−1) of A. citrulli. All coated seeds were dried for 20 min after mixing. Experiment was conducted by having two groups of seed coats (bacteria coated seeds and phage coated seeds) and normal seeds as a control group. Eight seeds of each group were sown in three pots (31.8 × 14.2 × 12.5 cm) containing 700 g autoclaved soil. After sowing seeds, three sets of treatment were performed. In the first set of treatment distilled water was added to pot containing phage coated seeds, pots coating bacteria coated seeds and normal seeds (total nine pots for this treatment). Second set of treatment conducted to determine the protective effects of the phage ACPWH in presence of bacteria in which 200 mL of phage suspension (106 PFU mL−1) was added to pots containing normal seeds and bacteria coated seeds (total six pots). Third set of treatment was done by adding 200 mL of bacteria (105 CFU mL−1) to each pots containing phage coated seeds and normal seeds. Schematic description of this experiment is presented as a supplementary data. All experimental plants were kept for 3 weeks, and germination and survival data were recorded. All experiments were conducted in triplicate.
Effect of phage coating on artificially infected seeds
Artificially infested seeds were produced by following the previously described method (Kubota et al. 2011). In short, commercially available watermelon fruits were purchased from Market and surface washing with DW and NaOH. One ml of A. citrulli suspension (\({10}^{6}\) CFU/ml) was injected to the watermelon fruit. Watermelon fruits were kept at 30℃ for 20 days. Then the seeds were harvested, surface washed and dried at room temperature. Protection activity of phage was examined through coating the infested seeds with bacteriophages by mixing seeds in SM buffer containing 106 PFU mL−1 phage ACPWH. Total six pots were set for the experiment in which three pots containing eight infested seeds with phage coat and other three pots with infested seeds. Number of seedlings were reordered after 2 weeks and experiments were repeated three times.
Statistical analysis
Analysis of variance (Anova) and Fisher`s LSD test were carried out by using the software SAS (SAS institute Inc., Cary, NC, U.S.A). LSD test was performed to compare the effect of phage treatments on the mean of seed germination and survival.
Results
Phage isolation and host range
A total of 200 leaf and soil samples from watermelon, cucumber, and pumpkin were collected from different parts of Korea. The presence of bacteriophage was detected in 100 of these samples by spot assay. After three cycles of plaque assays, a total of 46 pure bacteriophages were isolated. These phages were tested against 42 host bacterial stains to determine their host ranges, and a new bacteriophage named ACPWH (A. citrulli Phages with Wide Host) was selected for further study.
The host range of phage ACPWH is compared to that of the previously reported phage ACP17 in Table 1. Phage ACPWH lysed 39 of the 42 bacterial strains tested, including 10 strains that were resistant to phage ACP17. However, three strains, LB10-232, NWB-SC205, and KACC18649, were resistant to infection by both ACP17 and ACPWH. The infectivity of ACPWH to other bacteria, including human and plant pathogens, was also tested which none of the 40 tested bacteria were lysed by ACPWH.
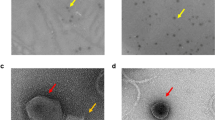
Electron microscopy
Phage ACPWH in lysate was concentrated by ultracentrifugation and observed with negative staining without further purification, to minimize morphological damage during purification. ACPWH has an icosahedral head and a long flexible tail, which is the typical morphology of the Siphoviridae family. The head of ACPWH is 55 ± 5 nm in width and 60 ± 5 nm in length, with a tail length of approximately 180 ± 5 nm (Fig. 1).
One-step growth curve
The infection cycle of phage ACPWH was analyzed by inoculating one of the sensitive host strains with ACPWH at a MOI of 0.01 and counting the phages in the solution using the double-layer agar method. The results showed a replication curve with three phases: latent phase, rise phase, and stationary phase. One-step growth curve analysis showed a typical curve with a 15 ± 5-min latent period. The highest titer was observed after 20 min of incubation, with a burst size of 189 PFU mL−1 (Fig. 2).
Temperature and pH stability
As shown in Fig. 3, ACPWH was highly stable after incubation for 3 days at − 80 °C and − 20 °C, with a slight decrease at 4 °C and relatively little change up to 50 °C (Fig. 3). There was a sharp decrease in phage titer at 55 °C, followed by a complete loss of infectivity at 60 °C. Meanwhile, the pH stability test of ACPWH showed almost no reduction in infectivity after 3 days of incubation at the pH range from 6 to 9. The phage titer decreased at pH 5.5, and no infectivity was observed below pH 5.5. In addition, ACPWH showed decreased infectivity at pH 10 and complete inactivation at pH 12.
Genome analysis of ACPWH
ACPWH genome was treated with DNase, RNase and Exonuclease III and, the corresponding band on an agarose gel disappeared in DNase and Exonuclease III treated sample, which indicated that ACPWH has a linear dsDNA genome. A genomic DNA library was constructed and analysis of the entire genome revealed that ACPWH has a genome of 42,499 bp with the GC content of 64.44%. The genome of ACPWH is predicted to encode 64 open reading frames (ORF) with no tRNA gene (Fig. 4). Length of ORFs ranges from 120 to 4077 bp which encode proteins of 39–1358 amino acid in size. Among all ORFs, 53 starts with of ATG as the start codon while 11 of ORFs start with GTG codon. Total 95.9% of the whole genome is covered by ORFs with total size of 40,776 bp. Out of 64 predicted ORFS, 30 ORFS are encoded by one strand and 34 ORFs are encoded by the other strand. Functional classification of the encoded proteins divided ORFs into three major groups where 44 ORFs encode hypothetical proteins, 8 ORFs encode phage structural proteins and rest of ORFs are related to the regulation of Phage DNA inside the host (Supplementary data Table 1)
Phast analysis showed that ACPWH has common genes with Ralstonia solanacearum Phage RS138, Bordetella bacteriophage BPP-1, Pseudomonas phage MD8, Pseudomonas phage phiMK, Stenotrophomonas phage S1, and Xylella phage Xfas53. Easyfig result confirmed that the phage ACPWH has at least 6 common genes with bacteriophage RS138. Alignment result of these two bacteriophages suggested that these two phages are mostly homologues in genes related to tail and tail fibers (Fig. 5). Complete genome sequence of ACPWH is submitted in NCBI genbank under the accession number MH727593.
Phylogenetic tree of bacteriophage ACPWH. The tree was generated based on aligned amino acid sequences using ClustalW. The percentage of replicate trees is shown next to the branches in which the associated taxa clustered together in the bootstrap test (1000 replicates). The branch lengths are number of substitution per sites. Mega 5 was used to generate the phylogenetic tree using the neighbor-joining method. *Family information of phages, S indicating Siphoviridae family and P indicating Podoviridae family of phages
Phylogenetic analysis of ACPWH with selected bacteriophages form siphoviridae and podoviridae family based on capsid protein amino acid sequence showed that ACPWH is located near Pseudomonas phage Psp6 and Pseudoalteromonas phage PHS21 with close relationship to Pseudoalteromonas phage PHS21 (Fig. 6).
In planta assay
The effects of phage ACPWH on watermelon plants in the presence of A. citrulli were determined in three sets of experiments. The protective effects of phage ACPWH were measured based on the germination and survival of watermelon seeds. Germination rate was determined 2 weeks after sowing and the survival rates of seeds were recorded 3 weeks after germination. As result, only 13% of normal watermelon seeds germinated when A. citrulli was added to the soil. The only germinated plant showed water-soaking symptoms on its cotyledon; full symptoms then developed, and the plant eventually died at 2 weeks (Fig. 7a). In contrast, seeds coated with bacteriophage ACPWH showed 95% germination when A. citrulli was inoculated to the soil (Fig. 8b). Bacteria coated seeds in soil treated with normal water had germination and survival rate of 13% and 10%, respectively. Addition of phage to pots containing bacteria coated seeds increase the germination rate to 96% and survival rate of 96% (Fig. 8c). Presence of phage in the soil or as seed coat showed no effect on germination or growth of normal watermelon seeds as well as seeds used in this experiment had 100% germination rate and survival rate.
Protective effects of ACPWH on the survival of watermelon plants in the presence of A. citrulli. a Untreated seeds in A. citrulli-inoculated soil. b Untreated seeds in ACPWH phage-treated soil. c Phage-coated seeds in A. citrulli-inoculated soil. d Bacterial-coated seeds in soil treated with ACPWH. Photos were taken 3 weeks after germination
Effects of bacteriophage ACPWH on the germination and survival rates of watermelon plants in the presence of Acidovorax citrulli. Error bars represent the standard deviation of three replicates. According to Fisher’s test of least significance difference (LSD), treatments with the same letters are not significantly different (P < 0.05). Capital letters represent the results of Fisher’s LSD for comparing the survival rate of watermelon seedling and lowercase letters represent the comparison of germination rate of watermelon seeds after treatments
Phage protection ability against artificially infested seeds
Bacteria infested seeds have shown 55% germination rate with symptomatic seedlings whereas phage coated seeds shown 88% germination rate. Germinated seedlings were kept for three more weeks after germination to compare the survival rate of watermelon plants. In result, seedlings from phage coated infected seeds were able to completely survive with 100% survival rate. In contrast to phage coated seeds, no treated seeds had approximately 15% of survival rate which survived plants fall off a month after germination (Fig. 9).
Discussion
Bacterial viruses have attracted attention generally due to limited strategies available for the management of bacterial diseases in plants. Despite, Phage biocontrol for Acidovorax citrulli, major threat for cucurbits, has not been studied well enough to be considered as a possible solution for controlling bacterial fruit blotch (BFB) (Goodridge 2004). Hence, in this study we searched for bacteriophages with wider host ranges comprising both genotype I and II strains. As shown in Table 1, the phage ACP17 was unable to lyse some genotype II strains, but the newly selected phage ACPWH infected all but three of these strains. One possible reason for the resistance is the source of the phages. In this study we have mostly collected the samples from watermelon farms. All three resistant strains belonged to group I, which is mainly isolated from non-watermelon hosts (Walcott et al. 2004). In contrast to its wide host range against A. citrulli strains, phage ACPWH did not cause lysis in any other bacteria tested. Although the number of bacteria tested was limited, this suggests that ACPWH will not affect normal bacterial populations in soil if used as a biological control agent in the future.
Result of sequencing confirmed that the phage has similar genome size of other Siphovirdae phages such as Staphylococcus phage StauST398-4 with genome size of 42,911 bp (Van Der Mee-Marquet et al. 2013). Bacteriophage ACPWH guanione/cyctonsine content is similar to the host genome (68.54%) same as phage Staphylococcus phage SpaA1 infecting Staphylococus pasteuri that has resembled G + C percentage to the host (Swanson et al.2012). Other related bacteriophages are known to be having relatively similar G + C percentage compare to the host bacteria that can be due to adaptation of phages to their hosts (Almpanis et al. 2018). Among Enzymes encoded by ACPWH genome, key factor for bacterial lysis is the phage hydrolase. Bacteriophage hydrolase can degrade carbohydrates in the bacterial cells (Loeffler et al. 2001). Other related enzyme named as helicase, relating to the unwinding of DNA and metabolism of the nucleuoacids which help phage genome during DNA replication (He et al. 2012). In addition, presence of protein gp09 part of thymidylate synthesis shown that phage DNA synthesize in infected host can be done with support of thymine which make proper phage infection and better burst size of phage ACPWH (Finer-Moore et al. 1994).
Phylogenetic tree analysis based on capsid protein was constructed using mega7 has shown similarity to bacteriophage that infects different host as ACPWH named of Pseudoalteromonas marina mano4. Despite, the amino acid size of capsid protein of the Pseudoalteromonas phage PHS21 is approximately 43 KDA while capsid protein of ACPWH is 37 kDA. This information suggesting that the evolution of the gene complement in ACPWH and the phylogenetically related to Pseudoalteromonas marina bacteriophages. In addition, phylogenetic result suggesting that bacteriophage ACPWH shared common ancestor with other phages named as Pseudomonas phage Psp6, Burkholderia phage AH2, Achromobacter phage phiAxp-2 and Loktanella phage pCB2051.
Bacteriophage RS138 gene encoding portal, lysis, lysin, tail, plate and protease are having high similarity among phage ACPWH. Thi et al. (2016) revealed that Phage RS138 belongs to Siphoviridae family with approximately similar genome size to ACPW. Size of the largest protein encoded by ACPWH is TOlA protein (1358 aa) which has a role in infection and integrity of outer membrane of host whereas RS138 largest encoded protein is tail type protein with size of 1565 aa larger than TolA in ACPWH. Despite the similarities between these two phages, phage RS138 has shown to have ORFs encoding putative genes for transcriptional regulator, transposase which is more similar to MU and MU like prophages that none have been seen in bacteriophage ACPWH. BLASTn, BLASTp and Rhast result available in this study suggesting that the phage ACPWH should be considered as new member of family Siphoviridae with genes related to closely related phages.
Bacteriophages can remain infective at different ion concentrations, temperatures, and pH levels (Ly-Chatain 2014) For example, bacteriophage λ remains stable in a wide pH range (3–11) (Jepson and March 2004), and bacteriophage PM2 remains infective at various pH levels, depending on the concentrations of NaCl and CaCl2 (Jończyk et al. 2011) this suggests that these factors can affect the attachment, penetration, and latent period of bacteriophages (Ly-Chatain 2014). ACPWH remained stable at high temperatures, similar to bacteriophage KSL-1 from the Siphoviridae family, whereas it has been reported that bacteriophages such as λ and T4, like bacteriophage Bp7, are no longer infective at very acidic (< pH 5) or very basic (> pH 10) pH levels, which was also observed in ACPWH. Meanwhile, watermelon plants are cultivated at temperatures ranging from 18 to 28 °C at farms, and up to 40 °C in greenhouses, at a pH range from 6.5 to 6.8. Furthermore, ACPWH was shown to be completely activated and infective under these conditions, indicating that ACPWH is a good candidate for phage biocontrol at farms as well as in greenhouses.
Seed health testing methods, such as detecting pathogens in contaminated seeds using the seedling grow-out assay, conventional PCR, real-time PCR, immunomagnetic separation of the pathogen followed by PCR, and loop-mediated isothermal amplification have been developed to detect A. citrulli in cucurbit seeds, but each method has limitations, such as the inhibition of PCR by seed-wash compounds or significant costs of equipment and reagents (Yan et al. 2019). Seed treatments such as fermentation, or treatment with HCl or peroxyacetic acid, can prevent seed transmission of BFB, but may adversely affect seed germination. None of the treatment methods is 100% effective, because they generally fail to eradicate bacteria from within the seed (Burdman and Walcott 2012a, b).
Coating seeds with biological agents can also reduce the appearance of BFB at an early stage. It has been reported that coating watermelon seeds with Bacillus strains can reduce the appearance of BFB (Li et al. 2015). Similarly, coating watermelon seeds with a microbial consortium or antagonistic Pseudomonas synxantha reduced the percentage of diseased seedlings by 27% and 5%, respectively, without affecting the germination rate (Giovanardi et al. 2015).
Seed coating and inoculation of phage to soil result showed that phage can be stable and protective in soil condition to reduce the BFB incidence. It is known that A. citrulli cause infection on watermelon plant in any stage of the plant growth after germination. Bin et al. (2013) showed that healthy seedling can be infected by A. citrulli in the farm or green house with high relative humidity and high-temperature condition needed for the plant growth. In addition, presence of A. citrulli in increase the incidence and transmission of the disease in farm or green houses. These studies suggesting that to control BFB outbreak, controlling method should be close to seeds and also protect the plant even after germination. Hence, phage ACPWH can provide be protective agent for the plant in the presence of A. citrulli in soil.
In this study, we evaluated the protective effects of ACPWH against BFB development by adding A. citrulli soil after untreated seeds were sown and coating seeds with A. citrulli. In both cases, due to presence of A. citrulli very low germination rates were observed. Latin et al. (1995) reported that covering seeds with over 103 CFU ml−1 of A. citrulli caused BFB symptoms, and A. citrulli can survive in the soil for a long time. Therefore, 2 × 107 CFU of A. citrulli in 700 g soil was used in this experiment, as it should be sufficient to cause BFB in the absence of ACPWH (as demonstrated in the control group). Therefore, 100% germination and survival in the presence of ACPWH suggests that this phage could be used to prevent BFB in soil containing A. citrulli. In addition, phage-coated seeds showed 96% germination even when 105 CFU ml−1A. citrulli was added to the soil (Fig. 8b), and none of the plants showed any BFB symptoms after 3 weeks (Fig. 7c).
Despite recent developments in the biocontrol of plant diseases using phages (Jones et al. 2007), the application of phages for the control of seed-borne diseases is very limited. Presence of bacteria under the seeds coat obliged us to seek for methods that assure protection effect of phage on infested seeds. As previously described method, artificially infested seeds can show the BFB symptoms as in this study, non-treated infested seeds showed low germination rate with symptomatic seedlings. In addition, compare to available strategies for seeds treatment bacteriophages are able to stay infective for long term around the seeds even after germination without any side effects on plants. Available seeds treatments such as heat and chemical treatments are mostly applied before sowing or after production of the seeds which due to location of the A. citrulli the treatment does not show 100% efficiency. However, Basit et al. (1992) reported that the nodulation of soybeans by desirable strains of rhizobia can be increased significantly by coating seeds with phages specific for undesirable strains. This evidence can help us to explain that phage coating provides close and long term interaction between the host and the phage which compare to other treatments provide a higher chance for controlling the disease.
Our results indicate that phages can be used as effective coating agents to prevent seed-borne diseases, by affecting the bacteria on the seeds or in nearby soil. It will also be possible to isolate multiple phage strains with different host ranges and use them as a cocktail to enhance the control efficiency (Kering et al. 2019). For phage ACPWH to function as a viable biocontrol agent, it must be able to treat the disease after it has developed on plants; further research on the effects of spraying ACPWH on plants with BFB symptoms is therefore underway.
References
Almpanis A, Swain M, Gatherer D, McEwan N (2018) Correlation between bacterial G+C content, 425 genome size and the G+C content of associated plasmids and bacteriophages. Microb Genom 4(4):e000168. https://doi.org/10.1099/mgen.0.000168
Basit HA, Angle JS, Salem S, Gewaily EM (1992) Phage coating of soybean seed reduces nodulation by indigenous soil bradyrhizobia. Can J Microbiol 38:1264–1269
Bonilla N, Rojas MI, Netto Flores Cruz G, Hung S-H (2016) Phage on tap–a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ 4:e2261
Burdman S, Walcott R (2012a) Acidovorax citrulli: generating basic and applied knowledge to tackle a global threat to the cucurbit industry. Mol Plant Pathol 13(8):805–815
Burdman S, Walcott R (2012b) Acidovorax citrulli: generating basic and applied knowledge to tackle a global threat to the cucurbit industry. Mol Plant Pathol 13:805–815
Curis E, Nicolis I, Moinard C, Osowska S (2005) Almost all about citrulline in mammals. Amino Acids 29:177–205
Czajkowski R, Ozymko Z, Lojkowska E (2014) Isolation and characterization of novel soilborne lytic bacteriophages infecting Dickeya spp. biovar 3 ('D. solani’). Plant Pathol 63:758–772
Databases | FAO | Food and agriculture organization of the united nations (2016)
Duckworth DH (1976) Who discovered bacteriophage? Bacteriol Rev 40:793–802
Eckshtain-Levi N, Munitz T, Živanović M, Traore SM (2014) Comparative Analysis of type III secreted effector genes reflects divergence of Acidovorax citrulli strains into three distinct lineages. Phytopathology 104:1152–1162
Ellis EL, Delbrück M (1939) The growth of bacteriophage. J Gen Physiol 22(3):365–384
Finer-Moore JS, Maley GF, Maley F, Montfort WR, Stroud RM (1994) Crystal structure of thymidylate synthase from T4 phage: component of a deoxynucleoside triphosphate-synthesizing complex. Biochemistry 33(51):15459–15468
Giovanardi D, Ferrari M, Stefani E (2015) Seed transmission of Acidovorax citrulli: implementation of detection in watermelon seeds and development of disinfection methods. In: VII Congress on Plant Protection Plant Protection Society of Serbia, pp 71–75
Goodridge LD (2004) Bacteriophage biocontrol of plant pathogens: fact or fiction? Trends Biotechnol 22:384–385
Guo S, Zhang J, Sun H, Salse J (2012) The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat Genet 45(1):51
Hassan Ema O, El-Meneisy ZA (2014) Biocontrol of halo blight of bean caused by Pseudomonas phaseolicola. J Virol 10:235–242
He X, Byrd AK, Yun MK, Pemble CW IV, Harrison D, Yeruva L, Dahl C, Kreuzer KN, Raney KD, White SW (2012) The T4 phage SF1B helicase Dda is structurally optimized to perform DNA strand separation. Structure 20(7):1189–1200
Hopkins DL, Thompson CM, Hilgren J, Lovic B (2003) Wet seed treatment with peroxyacetic acid for the control of bacterial fruit blotch and other seed-borne diseases of watermelon. Plant Disease 87(12):1495–1499
Jepson CD, March JB (2004) Bacteriophage lambda is a highly stable DNA vaccine delivery vehicle. Vaccine 22:2413–2419
Johnson KL, Minsavage GV, Le T, Jones JB, Walcott RR (2011) Efficacy of a nonpathogenic Acidovorax citrulli strain as a biocontrol seed treatment for bacterial fruit blotch of cucurbits. Plant Dis 95:697–704
Jończyk E, Kłak M, Międzybrodzki R, Górski A (2011) The influence of external factors on bacteriophages: review. Folia Microbiol (Praha) 56:191–200
Jones JB, Jackson LE, Balogh B, Obradovic A (2007) Bacteriophages for plant disease control. Annu Rev Phytopathol 45:245–262
Kering KK, Kibii BJ, Wei H (2019) Biocontrol of phytobacteria with bacteriophage cocktails. Pest Manag Sci 75(7):1775–1781
Kubota M, Hagiwara N, Shirakawa T (2011) A method to evaluate percentage of cucurbit seeds infested with Acidovorax avenae subsp. citrulli, pathogen of bacterial fruit blotch. J Gen Plant Pathol 77(2):112–115
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Latin R, Tikhonova I, Rane K (1995) Factors affecting the survival and spread of Acidovorax avenae subsp. citrulli in watermelon transplant production facilities. Phytopathology 85:1413–1417
Li L, Ge Y, Tian Y, Hu B (2015) Development of biological seed coating formulation for control of bacterial fruit blotch (BFB). J Agr Biotechnol 23(12):1649–1659
Loeffler JM, Nelson D, Fischetti VA (2001) Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294(5549):2170–2172
Ly-Chatain MH (2014) The factors affecting effectiveness of treatment in phages therapy. Front Microbiol 5:51
Mukerjee S (1963) The bacteriophage-susceptibility test in differentiating Vibrio cholerae and Vibrio eltor. Bull World Health Organ 28:333–336
Sambrook J, Russell DW (2006) Purification of nucleic acids by extraction with phenol: chloroform. Cold Spring Harb Protoc pdb-prot 4455.
Sharma KK, Singh US, Sharma P, Kumar A, Sharma L (2015) Seed treatments for sustainable agriculture—a review. J Appl Nat Sci 7:521–539
Swanson M, Reavy B, Makarova KS, Cock PJ, Hopkins DW, Torrance L, Koonin EV, Taliansky M (2012) Novel bacteriophages containing a genome of another bacteriophage within their genomes. PLoS ONE 7(7):40683
Thi BVT, Khanh NHP, Namikawa R, Miki K, Kondo A, Thi PTD, Kamei K (2016) Genomic characterization of Ralstonia solanacearum phage ϕRS138 of the family Siphoviridae. Arch Virol 161(2):483–486
Tian Y, Zhao Y, Wu X, Liu F (2015) The type VI protein secretion system contributes to biofilm formation and seed-to-seedling transmission of Acidovorax citrulli on melon. Mol Plant Pathol 16:38–47
Tlili I, Hdider C, Lenucci MS, Riadh I (2011) Bioactive compounds and antioxidant activities of different watermelon (Citrullus lanatus (Thunb.) Mansfeld) cultivars as affected by fruit sampling area. J Food Compos Anal 24:307–314
Walcott RW, Fessehaie A, Castro A (2004) Differences in pathogenicity between two genetically distinct groups of Acidovorax avenae subsp. citrulli on cucurbit hosts. J Phytol 152:277–285
Yamada T (2013) Filamentous phages of Ralstonia solanacearum: double-edged swords for pathogenic bacteria. Front Microbiol 4:325
Yan L, Zhao Y, Zhou J, Chen S (2019) Rapid and sensitive detection of Acidovorax citrulli in cucurbit seeds by visual loop-mediated isothermal amplification assay. J Phytol 167(1):10–18
Zivanovic M, Walcott R (2016) Further characterization of genetically distinct groups of Acidovorax citrulli strains. Phytopathology 107(1):29–35
Acknowledgements
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Agri-Bio Industry Technology Development Program funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (Grant #31702–4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors reported no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rahimi-Midani, A., Kim, JO., Kim, J.H. et al. Potential use of newly isolated bacteriophage as a biocontrol against Acidovorax citrulli. Arch Microbiol 202, 377–389 (2020). https://doi.org/10.1007/s00203-019-01754-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-019-01754-5