Abstract
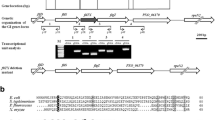
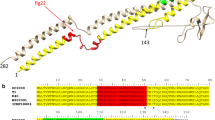
Genome annotation of the plant pathogen Xanthomonas axonopodis pv. citri (Xac), identified flagellar genes in a 15.7 kb gene cluster. However, FlgN, a secretion chaperone for hook-associated proteins FlgK and FlgL, was not identified. We performed extensive screening of the X. axonopodis pv. citri genome with the yeast two-hybrid system to identify a protein with the characteristics of the flagellar chaperone FlgN. We found a candidate (XAC1990) encoded by an operon for components of the flagellum apparatus that interacted with FlgK. In order to further support this finding, Xac FlgK and XAC1990 were cloned, expressed, and purified. The recombinant proteins were characterized by spectroscopic methods and their interaction in vitro confirmed by pull-down assays. We, therefore, conclude that XAC1990 and its homologs in other Xanthomonas species are, in fact, FlgN proteins. These observations extend the sequence diversity covered by this family of proteins.






Similar content being viewed by others
Abbreviations
- Xac :
-
Xanthomonas axonopodis pathovar citri
- 3AT:
-
3-Aminotriazole
- MM:
-
Molecular mass
- A:
-
Absorbance
- CD:
-
Circular dichroism
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
Aizawa SI (1996) Flagellar assembly in Salmonella typhimurium. Mol Microbiol 19:1–5
Aldridge P, Karlinsey J, Hughes KT (2003) The type III secretion chaperone FlgN regulates flagellar assembly via a negative feedback loop containing its chaperone substrates FlgK and FlgL. Mol Microbiol 49:1333–1345
Alegria MC, Docena C, Khater L, Ramos CHI, da Silva AC, Farah CS (2004) New protein–protein interactions identified for the regulatory and structural components and substrates of the type III secretion system of the phytopathogen Xanthomonas axonopodis pathovar citri. J Bacteriol 186:6186–6197
Alegria MC, Souza DP, Andrade MO, Docena C, Khater L, Ramos CHI, da Silva AC, Farah CS (2005) Identification of new protein–protein interactions involving the products of the chromosome- and plasmid-encoded type IV secretion loci of the phytopathogen Xanthomonas axonopodis pv. citri. J Bacteriol 187:2315–2325
Auvray F, Thomas J, Fraser GM, Hughes C (2001) Flagellin polymerization control by a cytosolic export chaperone. J Mol Biol 318:221–229
Bennett JCQ, Hughes C (2000) From flagellum assembly to virulence. Trends Microbiol 8:202–204
Bennett JCQ, Thomas J, Fraser G, Hughes C (2001) Substrate complexes and domain organization of the Salmonella flagellar export chaperones FlgN and FliT. Mol Microbiol 39:781–791
Blocker A, Komoriya K, Aizawa SI (2003) Type III secretion systems and bacterial flagella: Insights into their function from structural similarities. Proc Natl Acad Sci USA 100:3027–3030
Bohm R, Muhr R, Jaenicke (1992) Quantitative analyses of protein far UV circular dichroism spectra by neural networks. Protein Eng 5:191–195
Creasey EA, Delahay RM, Daniell SJ, Frankel G (2003) Yeast two-hybrid system survey of interactions between LEE-encoded proteins of enteropathogenic Escherichia coli. Microbiol 149:2093–2106
da Silva AC, Ferro JA, Reinach FC, Farah CS, Furlan LR, Quaggio RB, Monteiro-Vitorello CB, Van Sluys MA, Almeida NF, Alves LM, do Amaral AM, Bertolini MC, Camargo LE, Camarotte G, Cannavan F, Cardozo J, Chambergo F, Ciapina LP, Cicarelli RM, Coutinho LL, Cursino-Santos JR, El-Dorry H, Faria JB, Ferreira AJ, Ferreira RC, Ferro MI, Formighieri EF, Franco MC, Greggio CC, Gruber A, Katsuyama AM, Kishi LT, Leite RP, Lemos EG, Lemos MV, Locali EC, Machado MA, Madeira AM, Martinez-Rossi NM, Martins EC, Meidanis JC, Menck F, Miyaki CV, Moon DH, Moreira LM, Novo MT, Okura VK, Oliveira MC, Oliveira VR, Pereira HA, Rossi A, Sena JA, Silva C, de Souza RF, Spinola LA, Takita MA, Tamura RE, Teixeira EC, Tezza RI, Trindade dos Santos M, Truffi D, Tsai SM, White FF, Setubal JC, Kitajima JP (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417:459–463
Edelhock H (1967) Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 6:1948–1954
Elliot SJ, Hutcheson SW, Dubois MS, Mellies JL, Wainwright LA, Batchelor M, Frankel G, Knutton S, Kaper JB (1999) Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol Microbiol 33:1176–1189
Fahrner KA, Block SM, Krishnaswamy S, Parkinson JS, Berg HC (1994) A mutant hook-associated protein (HAP3) facilitates torsionally induced transformations of the flagellar filament of Escherichia coli. J Mol Biol 238:173–186
Finley BB, Falkow S (1997) Commom themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev 61:136–169
Francis MS, Aili M, Wiklund ML, Wolf-Watz WH (2000) A study of the Yop-LcrH interaction from Yersinia pseudotuberculosis reveals a role for hydrophobicity residues within the amphipathic domain of YopD. Mol Microbiol 38:85–102
Fraser GM, Bennett JCQ, Hughes C (1999) Substrate-specific binding of hook-associated proteins by FlgN and FliT, putative chaperones for flagellum assembly. Mol Microbiol 32:569–580
Gietz RD, Woods RA (2002) Transformation of yeast by the LiAc/SS carrier DNA/PEG method. Meth Enzymol 350:87–96
Gietz RD, Woods RA, Manivasakam P, Schiestl RH (1998) Growth and transformation of Saccharomyces cerevisiae. In: Spector D, Goldman R, Leinwand L (eds) Cells: a laboratory manual, culture and biochemical analysis of cells, vol I. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Gygi D, Fraser G, Dufour A, Hughes C (1997) Amotile but non-swarming mutant of Proteus mirabilis lacks FlgN, a facilitator of flagella filament assembly. Mol Microbiol 25:597–604
Honko AN, Mizel SB (2005) Effects of flagellin on inate and adaptive immunity. Immunol Res 33:83–101
James P, Halladay J, Craig EA (1996) Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425–1436
Jones CJ, Macnab RM, Okino H, Aizawa SI (1990) Stoichiometric analysis of the flagellar hook-(basal body) complex of Salmonella typhimurium. J Mol Biol 212:377–387
Journet L, Hughes KT, Cornelis GR (2005) Type III secretion: a secretory pathway serving both motility and virulence. Mol Membr Biol 22:41–50
Khater L, Alegria MC, Docena C, Santos TM, da Silva ACR, Ramos CHI (2005) In silico identification of potential chaperone genes that belong to types III and IV secretion systems in Xanthomonas axonopodis pv. citri. Gen Mol Biol 28:321–327
Kubori T, Shimamoto N, Yamaguchi S, Namba K, Aizawa S (1992) Morphological pathway of flagellar assembly in Salmonella typhimurium. J Mol Biol 226:433–446
Kutsukake K, Okada T, Yokoseki T, Lino T (1994) Sequence analysis of the flgA gene and its adjacent region in Salmonella typhimurium, and identification of another flagellar gene, flgN. Gene 143:49–54
Macnab RM (1999) The bacterial flagellum: reversible rotary propellor and type III export apparatus. J Bacteriol 181:7149–7153
Macnab RM (2003) How bacteria assemble flagella. Ann Rev Microbiol 57:77–100
Minamino T, Namba K (2004) Self-assembly and type III protein export of the bacterium flagellum. J Mol Microbiol 7:5–17
Moens S, Vanderleyden J (1996) Functions of bacterial flagella. Crit Rev Microbiol 22:67–100
Page AL, Fromont-Racine M, Sansonetti P, Legrain P, Parsot C (2001) Characterization of the interaction partners of secreted proteins and chaperones of Shigella flexneri. Mol Microbiol 42:1133–1145
Parchaliuk DL, Kirkpatrick RD, Agatep R, Simon SL, Gietz RD (1999) Yeast two-hybrid system: characterizing positives. Technical Tips Online 1:69:P01714 (Online) http://www.tto.trends.com
Silva JL, Miles EW, Weber G (1986) Pressure dissociation and conformational drift of the b-dimer of tryptophan synthase. Biochemistry 25:5780–5786
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nuc Acids Res 22:4673–4680
Uetz P, Giot L, Cagney G, Mansfield TA, Judson RS, Knight JR, Lockshon D, Narayan V, Srinivasan M, Pochart P, Qureshi-Emili A, Li Y, Godwin B, Conover D, Kalbfleisch T, Vijayadamodar G, Yang M, Johnston M, Fields S, Rothberg JM (2000) A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403:623–627
Vande Broek A, Vanderleyden J (1995) The role of bacterial motility, chemotaxis and attachment in bacteria-plant interactions. Mol Plant-Microbe Interact 8:800–810
Vonderviszt F, Imada K, Furukawa Y, Uedaira H, Taniguchi H, Namba K (1998) Mechanism of self association and filament capping by flagellar HAP2. J Mol Biol 284:1399–1416
Acknowledgments
We thank Nicola Conran for a critical reading of this manuscript. We are grateful for the excellent technical assistance of Silvia da Silva, Veruska Soares, and Ivanilce Guimarães. We thank Professor Stanley Fields for kindly providing the pOBD and pOAD plasmids and Professor Phillip James for kindly providing PJ694-a yeast cells. This study was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). LK, MCA, TMS, LT and CD thank FAPESP for fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jan Tommassen.
Rights and permissions
About this article
Cite this article
Khater, L., Alegria, M.C., Borin, P.F.L. et al. Identification of the flagellar chaperone FlgN in the phytopathogen Xanthomonas axonopodis pathovar citri by its interaction with hook-associated FlgK. Arch Microbiol 188, 243–250 (2007). https://doi.org/10.1007/s00203-007-0240-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-007-0240-y